Abstract
A 27-yr-old postgraduate student was found lying at the floor of an unsealed underground dry area, where a valve-opened empty cylinder of liquid nitrogen (150 L) was connected to a cap-removed empty Dewar-flask (10 L) via a copper infusion tube. No injury was found externally or internally. There were petechiae in the bilateral conjunctivae and periorbital skin. The dry area, measuring 300×130×260 cm, had a communication to the basement of the research building by a window measuring 90×60 cm in size at 130 cm above the floor. The scene reconstruction and atmosphere gas analysis revealed that the O2 concentration at 60 cm above the base dropped to 12.0% in 3 min and 10 sec, 10.0% in 8 min and 53 sec, 6.0% in 18 min and 40 sec, and 4.2% in 20 min and 28 sec. The primary cause of death was asphyxia by evaporated liquid nitrogen.
Nitrogen gas (N2), as a major inert gas of the atmosphere, is liquefied to liquid nitrogen (LN2) on cooling to -196℃ and easily vaporizes at room temperature. Known to be harmless with the exception of potential frostbite injury, LN2 is widely used in laboratories and in industry for freezing, filling, or cleaning purposes (1, 2). The few reported cases of accidental harm from LN2 (3-5) were caused by careless management, which may have involved sudden LN2 evaporation leading to oxygen depletion in limited spaces. However, those reports (3-5) did not reveal indisputable evidence of the cause of asphyxia even though they included complete autopsies, toxicological analysis, and blood gas analysis.
In this case report, a postgraduate student was found dead in an underground dry area where a cylinder of LN2 had been disposed. By a complete autopsy, we analyzed the atmosphere gas of the dry area through a reconstruction of the accidental scene in order to help define the cause of death.
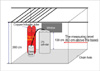
A 27-yr-old postgraduate student was found lying in a right lateral decubitis position on the latticed plastic pallets paving the floor of an underground dry area adjacent to a research building, approximately 12:00 p.m. on a day in June (temperature, 21.9-27.2℃; mean wind velocity, 2.4 m/sec; and mean humidity, 75.5%). Beside him was a cap-removed, empty Dewar-flask (10 L), connected to a valve-opened, empty cylinder of LN2 (150 L) via a copper infusion tube (Fig. 1). There was no leak defect in the cylinder, the infusion tube, or the Dewar-flask. The dry area, measuring 300×130×260 cm in dimensions, had a communication to the basement of the research building by a window measuring 90×60 cm in size at 130 cm above the base, as well as a drain hole located on the floor (Fig. 2). His last videogram was taken within his laboratory room at 07:17 a.m., when he was just going outside with the empty Dewar-flask in order to fill it with LN2. A college officer mentioned that the LN2 cylinder was fully filled on the day before the accident.
The autopsy was carried out 2 days later. No injury was found externally or internally. There were petechiae in the bilateral conjunctivae and periorbital skin (Fig. 3). Dark red livor mortis was seen in the dependent areas of the cadaver, and the heart was filled with dark red liquid blood. White froth was revealed in the lumens of the trachea and both bronchi. Neck dissection did not show any hemorrhage. Hyoid bone and thyroid cartilage were also intact. A few lymph nodes in the mesentery of the small intestine were slightly enlarged, determined to be reactive hyperplasia by histological examination. Congestion and edema were also noted in his organs without other pathomorphological alteration.
Scene reconstruction and atmosphere gas analysis (XP-302 II E, New Cosmos Electric Co., Osaka, Japan) were performed approximately 10:00 a.m., on a sunny day in September (temperature, 22.6-28.7℃; mean wind velocity, 3.5 m/sec; and mean humidity, 70.9%), approximately 2 months after the accident (Fig. 4). In order to reproduce the circumstances of the accident as closely as possible, concentrations of O2, CO, and H2S were measured at a level of 2 m below the ground and 60 cm above the base, while opening the valve of the cylinder filled with LN2, which was linked to an opened Dewar-flask. The O2 concentration dropped to 12.0% in 3 min and 10 sec, 10.0% in 8 min and 53 sec, 6.0% in 18 min and 40 sec, and 4.2% in 20 min and 28 sec (Fig. 5).
LN2 is used widely because it is cheap and innoxious. However, it is known that a small amount of LN2 can vaporize rapidly to large volumes of N2 (a liter of LN2 will produce 0.7 m3 of N2), and sudden evaporation of LN2 to N2 in a limited space can lead to O2 depletion in the air (2). If the O2 concentration of atmosphere drops below 10%, a human becomes unconscious immediately and unable rescue oneself from asphyxia (6). The dry area of this accident had been used to dispose the LN2 for 2 yr without the awareness of such danger, due to the misconceptions of both N2 being an innoxious gas and the dry area being an open space outside of the building. Therefore, it is a very important principle that LN2 must be disposed and used in an open and well-ventilated place (1, 2), and guidelines are needed to minimize the likelihood of injury and illness occurring from the use and storage of LN2.
Within the categories of asphyxia, this case can be treated as an environmental suffocation with no specific autopsy findings (7). However, external findings did include petechiae in the conjunctiva and periorbital skin, and internal findings included dark red liquid blood in the heart and congestion of visceral organs. Petechiae in the conjunctiva and periorbital skin of the decedent were unusual in that such petechiae in an asphyxiated cadaver generally suggests a sudden and strong break of venous return, as is commonly seen as a result of mechanical compression of the neck. However, the decedent did not show signs of any mechanical strain. This raises doubt as to whether petechiae are truly absent in cases of environmental suffocation (8). Frostbite was not present, owing to the plastic pallets at the base that left a gap between his body and the floor while LN2 was overflowing and evaporating. Enlargement of a few visceral lymph nodes might be reactive and self-limited.
Scene reconstruction was performed on a day in September, approximately 2 months later, with weather conditions similar to the day of the accident.
In conclusion, this is a noteworthy case highlighting the risk of environmental suffocation due to sudden evaporation of LN2 even in an open space, and the circumstances of death were eventually reproduced in scene reconstruction and confirmed by atmosphere gas analysis.
Figures and Tables
Fig. 1
The accident site in the underground dry area showed a LN2 cylinder and a Dewar-flask that were linked to a copper tube in front of two O2 tanks in a corner.

Fig. 2
The scheme of the accident site displayed general dimensions of the dry area, the sites of cylinder and Dewar-flask, and a window communicated of the basement of the building, and indicated the level we measured atmosphere gas proportion on scene reconstruction.
References
1. Air Products. Safetygram-7, Liquid Nitrogen. 1998. 1–4.
2. Safety Department, Imperial College London. Guidance Note 015, Liquid Nitrogen-Storage, Use and Transportation within College Premises. 2004. 1–15.
3. Gill JR, Ely SF, Hua Z. Environmental gas displacement: Three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002. 23:26–30.
4. Kernbach-Wighton G, Kijewski H, Schwanke P, Saur P, Sprung R. Clinical and morphological aspects of death due to liquid nitrogen. Int J Legal Med. 1998. 111:191–195.

5. Tabata N, Funayama M, Ikeda T, Azumi J, Morita M. On an accident by liquid nitrogen-histological changes of skin in cold. Forensic Sci Int. 1995. 76:61–67.
6. Wilkenfeld M. Rom WN, editor. Simple asphyxants. Environmental and occupational medicine. 1992. 2nd ed. USA: Lippincott Williams & Wilkins;535–538.
8. Di Maio VJM, Dana SE. Asphyxia. Handbook of Forensic Pathology. 1998. Texas, USA: Landes Bioscience;137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download