Abstract
The mechanism and cause of acute eosinophilic pneumonia are largely unknown. Many factors including the smoking of cigarettes have been suggested, but none have been proven to directly cause acute eosinophilic pneumonia. The authors report a case of acute eosinophilic pneumonia in a young Asian male who recently started smoking. The diagnosis was made based on his clinical course and results of chest radiography, lung spirometry, bronchoalveolar lavage, and transbronchial lung biopsies. After administration of methylprednisolone, his clinical course rapidly improved. A provocation test was designed to establish a connection between cigarette smoking and the development of acute eosinophilic pneumonia. After the provocation test, the patient showed identical symptoms, increase in sputum eosinophils, and worsening of pulmonary function. The results of the provocation test suggest that smoking may directly cause acute eosinophilic pneumonia, and support previous reports of cigarette smoking-induced acute eosinophilic pneumonia.
Acute eosinophilic pneumonia (AEP) is characterized by an acute febrile onset, progressive respiratory failure, bilateral chest infiltrates, an increase of eosinophils in bronchoalveolar lavage fluid (>25%), and a dramatic response to corticosteroids. It was first described by Allen and colleagues in 1989 (1), but the cause remains unknown. Some investigators suggest that unidentified inhaled antigens, such as Aspergillus species, mycotic infections, parasite infestations, drugs, and chemical agents, may induce a hypersensitivity reaction in an otherwise healthy individual (2).
The smoking of cigarettes has been proposed as a cause of AEP (3-5). Most of the papers published have described young Asians with episodic events, who started smoking prior to the time symptoms developed. We present a case of a young Korean patient, suspected to have cigarette smoking-induced AEP who was treated with corticosteroids and later showed recurrent symptoms on a provocation test.
An 18-yr-old Asian male presented to our department complaining of dyspnea on exertion and fever for over 24 hr. He denied chest pain, hemoptysis, or cough. He reported no family or past medical history and denied the use of drugs or medication. He denied any history of allergic conditions. He reported starting smoking up to 1 pack per day approximately 30 days prior to the admission. On examination, his body temperature was 38.5℃. The findings of complete blood cell counts revealed a white blood cell count of 27,800/µL with segmented neutrophils at 94%. Arterial blood gas analysis showed pH of 7.424, PaO2 of 66.2 mmHg, and PCO2 of 38.0 mmHg in room air. Inspiratory rhonchi and wheezing were heard on both lower lung fields on auscultation. Chest radiography showed multi-focal opacities in both lungs (Fig. 1). Blood and sputum cultures were taken, and antibiotics were promptly administered under the impression of bacterial pneumonia. Despite antibiotic therapy with moxifloxacin and clarithromycin, clinical and laboratory parameters did not improve. On the 3rd day of hospital admission, dyspnea worsened and he developed peripheral cyanosis. He also developed a productive cough. Expectorated sputum was mucoid with a whitish to yellowish appearance. Documented fever persisted, from 38 up to 39℃. Serial chest radiographs also showed aggravation (Fig. 1). Cultures from blood, sputum, and urine were negative, and serologic tests for Mycoplasma pneumoniae, Legionella pneumophila, Streptococcus pneumoniae, parasites (Taenia solium, Paragonomus westermani, Spirometra, Clonorchis sinensis), and viral tests including human immunodeficiency virus were also negative. Serologic markers for autoimmune diseases including immunoglobulin, complements and autoimmune antibodies were non-specific. High resolution computed tomography of the chest showed diffuse nonsegmental patchy or nodular ground glass attenuation in both lungs, small air space consolidations in the peripheral portion of both upper lungs, and diffuse interlobular septae thickening. On lung spirometry forced vital capacity (FVC), forced expiratory volume of 1 sec (FEV1) and their ratio (FEV1/FVC) were 65%, 54%, and 76% of predicted value, respectively. Diffusion of carbon monoxide (DLco) was 58% of predicted value.
Bronchoalveolar lavage (BAL) and transbronchial lung biopsies were performed on the 4th day of admission to clarify the pulmonary process. Analysis of BAL fluids showed a significant increase in eosinophils, 39%. Transbronchial lung biopsies revealed mild interstitial thickening with significant eosinophil infiltration (Fig. 3). The patient met the criteria for AEP described by Allen and Davis (2). Clinical symptoms rapidly subsided after administration of intravenous methylprednisolone (125 mg/day), and C-reactive protein and sputum eosinophil levels as well as follow-up chest radiology showed rapid improvement (Fig. 1). To identify the cause of AEP, we carefully reviewed the history of the patient. The only significant change in his life style or environment was the recent beginning of smoking 1 month before. To establish a connection between smoking and the development of AEP, we decided to perform a provocation test with given informed consent. He was given 1 cigarette to smoke, followed by an additional 3 cigarettes 2 hr later. Before and after smoking, his vital signs, sputum eosinophil percentage, C-reactive protein, white blood cells with % of eosinophils from peripheral blood, partial oxygen pressure, spirometry, and DLCO were checked regularly. The same laboratory and lung function parameters were followed up serially when the patient perceived any changes in his condition. Two hours after the last cigarette smoking, a mild fever at 37.6℃ developed. High fever and chills developed 7 hr later. Cough and sputum redeveloped the following day. An increase in self expectorated sputum eosinophils and decrease in FEV1 and DLCO (Fig. 2) were observed. Fever and other symptoms were relieved by methylprednisolone.
The diagnostic criteria for AEP was suggested by Allen and Davis (2), which include an acute febrile illness of short duration (usually less than one week), hypoxemic respiratory failure, diffuse pulmonary opacities on chest radiograph, BAL eosinophilia >25 percent, evidence of eosinophilic infiltrates (acute and organizing diffuse alveolar damage with prominent eosinophilia) on lung biopsy, and absence of known causes of eosinophilic pneumonia, including drugs, infections, asthma, or atopic disease. Some authors suggest that the diagnosis can be made with confidence without lung biopsy in immunocompetent patients with a compatible history and prominent BAL eosinophilia in the absence of infection or other know precipitants (6). The patient in our case met the criteria, and we were able to make the diagnosis of AEP without a difficulty. However, we were not certain whether smoking played a role in the development of AEP. This led us to design a provocation test.
Since 1996, there have been several reports of cigarette smoking-induced AEP. Some investigators have tried a cigarette smoking challenge test to provoke recurrence in patients who recovered from AEP (3). The cigarette smoking challenge test that we designed was similar, but the test was performed almost immediately after the patient showed full recovery. We demonstrated that not only sputum eosinophilia but the symptoms of AEP and worsening of pulmonary function could be reproduced by a provocation test. The patient's symptoms after the provocation test were mild, but almost identical to those at admission. Worsening of pulmonary function, particularly DLCO, and the increase in self expectorated sputum eosinophils were also characteristic. Moreover, symptoms and pulmonary function normalized almost immediately after readministration of steroids. Although symptoms fully recovered, peripheral eosinophil counts were beginning to climb at the time we decided to perform the provocation test. However, the absence of peripheral eosinophilia in the acute stages of AEP with elevation during recovery is a feature of AEP that has been reported in several studies (7-10). Because peripheral eosinophilia was also absent in the acute stages of disease and appeared after the patient had shown recovery, we decided to perform the test. Additionally, we could find no previous reports of febrile episodes following provocation at the time of recovery when peripheral eosinophilia was evident (3, 10).
Despite his recurrent symptoms, we could not find significant chest radiographic changes following the provocation test. Generally, the early radiographic findings of AEP are faint reticular markings that represent interstitial infiltration. As the disease progresses and/or the patient is exposed to continuous stimuli, diffuse interstitial and patchy alveolar infiltrates develop (8). We can only speculate that although the single stimulus of the provocation test was enough to induce a decrease in DLco and an inflammatory febrile response, it may not have been sufficient enough to induce chest radiographic changes. To our knowledge, there have been no reports of chest radiographic change after a provocation test in patients with AEP in the literature written in English.
After discharge, the patient restarted cigarette smoking, but showed no evidence of recurrence for more than 1 yr of follow-up. This may be explained by the development oftolerance. There have been several reports of tolerance following resumption of smoking cigarettes, but the mechanism remains unknown (4). Shintani et al. speculated that desensitization to cigarette smoke or interaction of various drugs and viruses with cigarette smoke may play a role in the development of tolerance (4). Indeed, though several investigators designed provocation tests to verify smoking-induced AEP, tests that were not associated with recurrent symptoms after provocation were performed 10-30 days after recovery (3, 10). Considering this, the performance of the provocation test immediately after the patient's recovery and associated recurrent symptoms may suggest that tolerance develops earlier than we expected.
To date, the cause or mechanism of AEP is not completely understood, and no single organism or agent has been proved to directly cause AEP. Some patients have been involved in unusual outdoor activities just prior to the onset of their illness (6, 7, 11, 12). The possible role of environmental factors at home has also been suggested (13). High levels of interleukin-5 have been found in the BAL fluid (14-16) suggesting its pathogenic role in AEP. In addition, interleukin-1 receptor antagonist, soluble type II interleukin-1 receptor, vascular endothelial growth factor, and granulocyte macrophage colony stimulating factor have also been found in the BAL fluid of patients with AEP, but their roles in the pathogenesis of AEP remain unknown (16, 17).
The cause of AEP is unknown and cigarette smoking may represent only one of many associated factors. However, the recurrence of eosinophlic pneumonia by provocation test supports previous reports that in certain individuals cigarette smoking may induce AEP. Further studies and understanding of the mechanism of AEP are needed to reach a conclusion, and the use of provocation tests such as the one used in our case may shed a light on this subject. The majority of reported cigarette smoking-induced AEP cases are young adults who were first-time smokers. In light of these reports and considering the increase of early cigarette smoking in young adults, it is important that physicians are aware of the possibility of this potentially life-threatening, but fully reversible disease.
Figures and Tables
Fig. 1
The time axis of sputum eosinophil percentage, absolute eosionphil count per microliter, chest radiograph, and high resolution computed tomography findings. Administration of 125 mg/day of methylprednisolone is shown by short arrows. The cigarette smoking challenge test is shown by the long arrow. Multifocal patchy infiltrates and interlobular septae thickening can be seen on chest radiography and high-resolution computed tomography. After administration of methlyprednisolone, the infiltrates and elevated sputum eosinophil percentage rapidly resolved. After the cigarette smoking challenge test, a sharp increase of in sputum eosinophils was observed, but there were no recurrent findings of lung infiltrates on chest radiography.
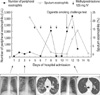
Fig. 2
The cigarette smoking challenge test and changes in body temperature, forced vital capacity (FVC), forced expiratory volume of 1 sec (FEV1), and diffusion of carbon monoxide (DLco) with respect to the elapsed time after smoking. Approximately 3 hr after smoking, a moderate decrease in DLco and mild decrease in FVC and FEV1 were observed. Body temperature reached its peak 6 hr after the first cigarette smoking.

References
1. Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989. 321:569–574.

3. Watanabe K, Fujimura M, Kasahara K, Yasui M, Myou S, Kita T, Watanabe A, Nakao S. Acute eosinophilic pneumonia following cigarette smoking: a case report including cigarette-smoking challenge test. Intern Med. 2002. 41:1016–1020.

4. Shintani H, Fujimura M, Ishiura Y, Noto M. A case of cigarette smoking-induced acute eosinophilic pneumonia showing tolerance. Chest. 2000. 117:277–279.

5. Nakajima M, Manabe T, Niki Y, Matsushima T. Cigarette smoke-induced acute eosinophilic pneumonia. Radiology. 1998. 207:829–831.

6. Pope-Harman AL, Davis WB, Allen ED, Christoforidis AJ, Allen JN. Acute eosinophilic pneumonia: a summary of 15 cases and a review of the literature. Medicine (Baltimore). 1996. 75:334–342.
7. Philit F, Etienne-Mastroianni B, Parrot A, Guerin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia; a study of 22 patients. Am J Respir Crit Care Med. 2002. 166:1235–1239.
9. King MA, Pope-Harman AL, Allen JN, Christoforidis GA, Christoforidis AJ. Acute Eosinophilic pneumonia: radiologic and clinical features. Radiology. 1997. 203:715–719.

10. Shiota Y, Kawai T, Matsumoto H, Hiyama J, Tokuda Y, Marukawa M, Ono T, Mashiba H. Acute eosinophilic pneumonia following cigarette smoking. Intern Med. 2000. 39:830–833.

11. Rom WN, Weiden M, Garcia R, Yie TA, Vathesatogkit P, Tse DB, McGuinness G, Roggli V, Prezant D. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002. 166:797–800.

12. Badesch DB, King TE Jr, Schwartz MI. Acute eosinophilic pneumonia: a hypersensitivity phenomenon? Am Rev Respir Dis. 1989. 139:249–252.

13. Imokawa S, Sato A, Hayakawa H, Toyoshima M, Taniguchi M, Chida K. Possible involvement of an environmental agent in the development of acute eosinophilic pneumonia. Ann Allergy Asthma Immunol. 1996. 76:419–422.

14. Taniguchi H, Kadota J, Fujii T, Matsubara Y, Katoh S, Mukae H, Matsukura S, Kohno S. Activation of lymphocyte and increased interleukin-5 levels in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. Eur Respir J. 1999. 13:217–220.
15. Yamaguchi S, Okubo Y, Hossain M, Fujimoto K, Honda T, Kubo K, Sekiguchi M, Takatsu K. IL-5 predominant in bronchoalveolar lavage fluid and peripheral blood in a patient with acute eosinophilic pneumonia. Intern Med. 1995. 34:65–68.

16. Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996. 97:1366–1374.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download