Abstract
To enhance the accuracy for determining the precise localization, the findings of the compound nerve action potentials (CNAPs) of the common peroneal nerve (CPN) were investigated in patients with common peroneal mononeuropathy (CPM) in the knee, and the sural sensory nerve action potentials (SNAPs) were also analyzed. Twenty-five patients with CPM in the knee were retrospectively reviewed. The findings of the CNAPs of the CPN recorded at the fibular neck and the sural SNAPs were analyzed. The lesion was localized at the fibular head (abnormal CNAPs) and at or distal to the fibular head (normal CNAPs). Seven patients were diagnosed as having a lesion at or distal to the fibular neck, and 18 cases were diagnosed as having a fibular head lesion. The sural SNAPs were normal in all the cases of lesion at or distal to the fibular neck. Among 18 cases of fibular head lesion, the sural SNAPs were normal in 7 patients: two cases of conduction block and 5 cases of mild axon loss. Eleven patients showed abnormal sural SNAPs. Of those, 9 cases were severe axon loss lesions and 2 patients were diagnosed as having severe axon loss with conduction block. The recording of the CNAPs may enhance precise localization of CPM in the knee. Moreover, the sural SNAPs could be affected by severe axonal lesion at the fibular head.
Common peroneal neuropathy (CPM) resulting in foot drop is a common clinical entity. A vast majority of peroneal neuropathy cases occur along the common peroneal nerve (CPN) that passes around the fibular head (1). Most of these lesions are traumatic in origin due to laceration, traction, and especially compression (2, 3). Recording the compound muscle action potential (CMAP) over the extensor digitorum brevis or the tibialis anterior is performed to determine the localization or severity of the lesion (3-8). However, using this approach, we may not be able to determine the lesion's location if the focal lesion of the peroneal nerve is located at or distal to the fibular neck area (3). Lee proposed that recording the compound nerve action potential (CNAP) of the CPN at the fibular neck may help to determine the precise location of a peroneal nerve lesion in the knee (fibular head lesion vs lesion at or distal to the fibular neck) (3).
The CPN commences from the lower part of the thigh and it courses downward along the lateral border of the popliteal fossa to reach the back of the head of the fibula. It winds round the neck of the fibula, whereupon it divides into the superficial and deep peroneal nerves (9). As the CPN passes through the popliteal fossa, it gives off two branches. One of these is the lateral cutaneous nerve of the calf, and its distribution is on the anterolateral aspect of the proximal leg. The other is the peroneal anastomotic nerve, which, after passing over the lateral head of the gastrocnemius muscle, extends to the middle third of the leg, where it joins the anastomotic (communicating) branch of the tibial nerve to form the sural nerve (Fig. 1) (9, 10). Thus, the sural sensory nerve action potentials (SNAPs) could be affected in certain cases of common peroneal nerve lesions.
To enhance the accuracy of precise localization, we investigated the findings of the CNAPs of the CPNs recorded at the fibular neck in patients with CPM in the knee, and the sural SNAPs were analyzed according to the location and severity of the lesion in these patients.
Thirty-two patients diagnosed with non-traumaic CPM were retrospective reviewed. The patients with diabetes mellitus or polyneuropathy were excluded. The patients who undertook the electrodiagnostic examination before 7 days after onset were also excluded. Finally, 25 patients with CPM in the knee were included. The delay between the onset of symptom and electrophysiologic evaluation was 10 days to 4 months. The studies conducted on the patients were segmental motor conduction study of the common peroneal nerve (ankle-fibular head-popliteal fossa), tibial motor nerve conduction study, and sensory conduction studies of the common peroneal mixed nerve, the superficial peroneal nerve, and the sural nerve. Needle EMG of the lower extremity muscles was also performed.
Common peroneal CMAPs was recorded over extensor digitorum brevis (EDB). If the common peroneal CMAP was not evoked with EDB recording, CMAP was recorded over tibialis anterior (TA). All the recordings of SNAPs were studied with E1 and E2 electrode embedded in a plastic bar. For recording the CNAPs of the CPNs, the active recording electrode was secured just distal to or at the fibular neck area, whereas the stimulating electrode was placed on the medial border of the lateral hamstring tendon at the level of the popliteal crease (Fig. 2) (3, 11). The superficial peroneal SNAP for the intermediate branch was recorded at 1 to 2 cm medial to the lateral malleolus at the ankle, and the nerve was stimulated at 12-14 cm proximal to the recording electrode, just anterior to the anterior edge of the fibula (11). The sural SNAP was recorded posterior to the lateral malleolus, while the nerve was stimulated at 14 cm proximal to the recording electrode in the posterior aspect of the leg. The onset latency and baseline-to-peak amplitude were bilaterally measured (11). An amplitude of SNAPs less than half of the normal side was interpreted as abnormal.
The CPM was diagnosed if the electrodiagnostic findings fulfilled one of common peroneal motor conduction study with or without abnormal superficial SNAP and needle electromyographic findings (1, 12): 1) absent or low CMAP with fibular head stimulation in EDB and/or TA recordings, 2) a decrease in CMAP negative potential amplitude from fibular head to popliteal fossa greater than 20% with or without an segmental slowing of fibular head-popliteal fossa segment (greater than 10 m/sec slower than ankle to fibular head segment), 3) significant change in CMAP configuration at the popliteal fossa site compared to the fibular site with or without a segmental slowing of fibular head-popliteal fossa segment (>10 m/sec than ankle to fibular head segment), 4) absent or low superficial SNAP, and 5) abnormal spontaneous activities and/or polyphasic motor unit potentials (MUPs) with reduced recruitment patterns in common peroneal innervated muscles.
The lesion was localized according to the findings of the CNAPs of the CPNs. The normal value of CNAPs of the CPN was referenced to the value of a previous study at our electrodiagnostic laboratory (3): amplitude, 24.8±7.4 µV; velocity, 61.6±4.5 m/sec. If the potential was not evoked or if it was of low amplitude, then the lesion was localized at the fibular head and was diagnosed at or distal to the fibular neck in the cases with a normal CNAP of CPN (3). The sural SNAPs were evaluated in each lesion. To investigate the relation of the severity of the lesion and the sural SNAP, the pathophysiology of the fibular head lesion was classified into three patterns (conduction block, axon loss, and mixed axon loss/conduction block) (1): conduction block, a decrease in CMAP or significant change in CMAP configuration with or without segmental slowing of fibular head-popliteal fossa segment, normal superficial SNAP, and normal or polyphasic MUPs with reduced recruitment patterns; axon loss, absent or low CMAP with fibular head stimulation, absent or low superficial SNAP, and abnormal spontaneous activities and/or polyphasic MUPs with reduced recruitment patterns.
The CNAPs of the CPNs were normal in 7 cases, and in 18 cases, the amplitude was of low amplitude or showed no response. Thus, 7 cases were diagnosed as lesions at or distal to the fibular neck and 18 cases were diagnosed as fibular head lesions (Fig. 3, Table 1).
The sural SNAPs were normal in all the cases that had a lesion at or distal to the fibular neck. Among the 18 cases of fibular head lesion, the sural SNAPs were normal in 7 patients, of which the lesions demonstrated conduction block in 2 cases and mild axon loss in 5 cases. Eleven patients showed abnormal sural SNAPs. Of these, 9 cases demonstrated severe axon loss lesions and 2 patients were diagnosed as severe axon loss with conduction block (Fig. 3, Table 2).
The CPN courses downward and inferolaterally from the apex of the popliteal fossa to the back of the fibular head (13). The nerve passes deep to the biceps femoris and, it is technically difficult to stimulate it above the popliteal fossa. Because the nerve is superficial at the point where the medial border of the lateral hamstring tendon meets with the popliteal crease, it is, therefore, easily stimulated (3). CPM can occur as the nerve passes beneath the biceps femoris tendon in the popliteal fossa (14), over the bony prominence of the fibular head and in the fibular tunnel formed by the origin of the peroneus longus muscle and the intermuscular septum (13, 15). The second fibro-osseous canal, 4 cm distal to the first one, formed by the origin of the extensor digitorum longus muscle may also rarely constrict the deep peroneal nerve (13).
Since the pathology is readily determined by electrodiagnosis, electromyography and detailed nerve conduction study can be extremely valuable in establishing the diagnosis and location of CPM and for determining the type of pathology and, thus, the treatment plan and the prognosis (16). To determine the location and severity of CPM, conduction studies with stimulation of the nerve distal to the fibular head and popliteal fossa and recording from the extensor digitorum brevis are usually performed (3). Decrement of the conduction velocity across the fibular head, a drop of the amplitude of the CMAPs, or both can be observed (3). Patients with foot drop require conduction studies of the CPN; the recording electrodes should be placed on the extensor digitorum brevis and tibialis anterior. Conduction block is the common pathophysiology of the lesions (3). Katirji and Wilborun reported that recording the tibialis anterior muscle was the single most important electrophysiologic study; it localized all 52 lesions causing conduction block at the fibular head. In contrast, the peroneal motor velocity along the knee-to-fibular head segment showed slowing in only five of the 52 cases (16). Kanakamedala et al. conducted short segment stimulation (SSS) at 2 cm intervals over a 10 cm distance across the knee. Whereas only 9 patients out of 18 showed slowing of motor conduction velocity across the 10 cm segment, 14 patients showed significant reduction of the amplitude and prolongation of the conduction time in one or more short segments. Thus, they postulated that the SSS technique is a sensitive and reliable procedure for the detection of mild compression or entrapment of the peroneal nerve around the knee (17). Kanakamedala et al. have also reported that a majority of the lesions were located just proximal to the fibular head (17), which is in contrast to the findings of Brown and Yates who reported that the maximally reduced conduction velocity was most often detected just distal to the fibular head (18).
Lee first described a technique for recording the CNAP of the CPN and reported 2 cases of common peroneal neuropathy at the knee with achieving precise localization by using this method (3). The CNAP of the CPN was normal in one case with a lesion at or distal to the fibular neck, while the potential was unobtainable in another case of fibular head lesion. In the present study, 7 patients out of 25 were diagnosed as having a lesion at or distal to the fibular neck and 18 patients were diagnosed as having a fibular head lesion based on the findings of the CNAPs of the CPN. Nine patients out of 18 patients with fibular head lesion revealed abundant fibrillation potentials and no motor unit potential on needle electromyography, whereas all the patients with a lesion at or distal to fibular neck showed single to discrete or reduced recruitment patterns on needle EMG. Based on these findings, we could predict favorable outcome in patients with a lesion at or distal to fibular neck.
The sural nerve originates from both the tibial and peroneal nerves. A recent study reported that the relative contribution of medial sural cutaneous nerve from the tibial nerve was 37% higher than that of peroneal anastomotic nerve (19). Wilborun described that the fibers evaluated by the sural nerve conduction study are apparently derived solely from the posterior tibial contribution, as the sural amplitudes are characteristically normal with axon loss peroneal mononeuropathy (1). However, in the present study, 11 out of 18 patients with fibular head lesion showed low sural SNAPs, while 7 patients with fibular head a lesion and 7 cases with a lesion at or distal to the fibular neck revealed normal sural SNAPs. Although the peroneal anastomotic nerve branches as the CPN passes through the popliteal fossa, the amplitude of the sural SNAP could be reduced because of retrograde degeneration in CPM with fibular head lesions that is relatively proximal to the lesions at or distal to the fibular neck. The pathophysiology of 18 patients with fibular head lesions in this study was as follows: 11 patients with low sural SNAPs had severe axon loss with or without conduction block, and 7 patients with normal sural SNAP had conduction block in 2 cases and mild axon loss in 5 cases. Why the patients revealed normal findings of sural SNAPs in fibular head lesions might be due to the pathophysiogy of the lesion that was conduction block or mild axon loss and also could be due to the higher proportion of medial sural cutaneous nerve in sural nerve formation.
Based on the results of this study, considering the peroneal anastomic nerve to the sural nerve, the sural SNAP could be affected by CPM at the fibular head, especially in the lesions of severe axon loss. Therefore, considering these factors, electrophysiologic diagnosis of coexisting incomplete sural neuropathy other than CPM should not be included in the interpretation of axona loss lesions at the fibular head.
In summary, the recording of the compound nerve action potential of the common peroneal nerve, in addition to the conventional methods, may enhance the differentiation of fibular head lesion from a lesion at or distal to the fibular neck in the diagnosis of CPM at the knee. Moreover, the fact that the sural SNAP could be affected by severe axon loss lesions at the fibular head should be considered in the interpretation of CPN.
Figures and Tables
Fig. 2
Common peroneal mixed nerve conduction study across the fibular head (with permission, courtesy of Prof. Hang J. Lee).
1, Common peromeal nerve (N); 2, common fibular N; 3, superficial peroneal N; 4, sural N.
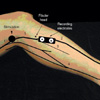
Fig. 3
Location and pathophysiology of the lesion according to the findings of the compound nerve action potential of common peroneal nerve and the sural sensory nerve action potential in patients with common peroneal mononeuropathy in the knee.

References
1. Wilbourn AJ. AAEE case report #12: Common peroneal mononeuropathy at the fibular head. Muscle Nerve. 1986. 9:825–836.

2. Goldner JC, Thomas JE. Foot drop. GP. 1969. 40:89–96.
3. Lee HJ. Compound nerve action potential of common peroneal nerve recorded at fibular neck: its clinical usefulness. Am J Phys Med Rehabil. 2001. 80:108–112.
4. Devi S, Lovelace RL, Duarte N. Proximal peroneal nerve conduction velocity: recording from anterior tibialis and peroneus brevis muscles. Ann Neurol. 1977. 2:116–119.
5. Singh N, Behse F, Buchthal F. Electrophysical study of peroneal palsy. J Neurol Neurosurg Psychiatry. 1974. 37:1202–1213.
6. DeLisa JA, Lee HJ, Baran EM, Lai KS, Spielholz N. Manual of nerve conduction and clinical neurophysiology. 1994. 3rd ed. New York: Raven Press;118–127.
7. Campbell WW. AAEM Course D: Fundamentals in electrodiagnostic medicine: electromyograhy and conduction studies in the diagnosis and management of entrapment syndromes. 1993. 37–40.
8. Robinson LR. Johnson EW, Pease WS, editors. Entrapment neuropathies and focal neuropathies. Practical Electromyography. 1997. Baltimore: Williams & Wilkins;262–267.
9. Haymaker W, Woodhall B. Peripheral nerve injuries, Principles of diagnosis. 1953. Philadelphia & London: WE Saunders Co;287–30.
10. Netter FH. The Ciba collection of medical illumstrations: Musculoskeletal system. Part I Anatomy, physiology and metabolic disorders. 1987. Summit, New Jersey: Ciba-Geigy;82.
11. Lee HJ, Delisa JA. Nerve conduction study and surface anatomy for needle electromyography. 2005. 4th ed. Philadelphia: Lippincott Williams & Wilkins;78–79.
12. Dumitru D. Dumitru D, editor. Focal peripheral neuropathies. Electrodiagnostic Medicine. 1996. Philadelphia: Hanley & Belfus;898–904.

13. Berry H, Richardson PM. Common peroneal nerve palsy: a clinical and electrophysiological review. J Neurol Neurosurg Psychiatry. 1976. 39:1162–1171.

14. Sunderland S. Nerve and Nerve Injuries. 1978. 2nd ed. Baltimore: WB Saunders;925–991.
15. Stewart JD, Aguayo AJ. Dyck PJ, Thomas PK, Lambert EH, Bunge R, editors. Compression and entrapment neuropathies. Peripheral Neuropathy. 1984. 1st ed. Philadelphia: WB Saunders Co;1435–1457.
16. Katirji MB, Wilbourn AJ. Common peroneal mononeuropathy: a clinical and electrophysiologic study of 116 lesions. Neurology. 1988. 38:1723–1728.

17. Kanakamedala RV, Hong CZ. Peroneal nerve entrapment at the knee localized by short segment stimulation. Am J Phys Med Rehabil. 1989. 68:116–122.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download