Abstract
NAD(P)H oxidase plays an important role in hypertension and its complication in aldosterone-salt rat. We questioned whether NAD(P)H oxidase subunit expression and activity are modulated by aldosterone and whether this is associated with target-organ damage. Rats were infused with aldosterone (0.75 µg/hr/day) for 6 weeks and were given 0.9% NaCl±losartan (30 mg/kg/day), spironolactone (200 mg/kg/day), and apocynin (1.5 mM/L). Aldosterone-salt hypertension was prevented completely by spironolactone and modestly by losartan and apocynin. Aldosterone increased aortic NAD(P)H oxidase activity by 34% and spironolactone and losartan inhibited the activity. Aortic expression of the subunits p47phox, gp91phox, and p22phox increased in aldosterone-infused rats by 5.5, 4.7, and 3.2-fold, respectively, which was decreased completely by spironolactone and partially by losartan and apocynin. Therefore, the increased expression of NAD(P)H oxidase may contribute to cardiovascular damage in aldosterone-salt hypertension through the increased expression of each subunit.
Growing evidence has emerged to show that aldosterone plays an independent role in the development of cardiovascular organ damage. Aldosterone/salt imbalance is detrimental to patients with hypertension (1), atherosclerosis (2), and heart failure (3) and it can lead to progressive tissue damage in the heart, vasculature, and kidneys (1). The role of aldosterone in the pathogenesis of cardiovascular disease in humans was convincingly established by results of the Randomized Aldactone Evaluation Study (RALES) (3), and the EPlerenone neuroHormonal Efficacy and SUrvival Study (EPHESUS)(4). The mechanism by which aldosterone dysregulation may contribute to cardiovascular disease is complex. Several factors have been proposed to explain the negative effects of aldosterone on cardiac fibroblasts and myocytes, and vascular endothelial and smooth muscle cells of the cardiovascular system, including pro-inflammatory and pro-oxidative properties (5). The pro-inflammatory and pro-fibrotic effect of aldosterone induces target cell and organ to be damaged structurally, functionally and mechanically, in particular due to aldosterone induced oxidative stress via modulation of NAD(P)H oxidase (6).
Activation of vascular NAD(P)H oxidase is a major source of vascular reactive oxygen species (ROS). We and others have demonstrated that increased ROS is associated with aldosterone-mediated cardio, renal, and vascular damage in rats (7-10). Aldosterone has a direct effect on oxidative stress through its ability to increase the levels of p22phox, an major subunit of NAD(P)H oxidase, essential for superoxide anion generation (9, 11). Further, gp91phox and 3-nitrotyrosine in heart (7, 8), p22phox, Nox-4, and gp91phox in kidney (10), and p22phox in aorta (9) were increased in aldosterone/salt rats. Therefore, these data suggest the possibility that at least some of the aldosterone-salt-induced ROS production in the target organs are mediated through the NAD(P)H oxidase pathway. Most studies were performed in in vitro conditions. In the present study, we questioned whether NAD(P)H oxidase subunit expression and activity are modulated by aldosterone in vivo and assessed whether this is associated with target-organ damage in aldosterone-dependent hypertension.
The study was conducted according to recommendations of the Animal Care Committee of the Samsung Biomedical Research Institute and Use Committee. Male Sprague-Dawley rats (Charles River Laboratory, Yokohama, Japan), aged 8 weeks and weighing 250 g were studied. Sham-operated rats served as control. Rats underwent right uninephrectomy via flank incision. In sham-operated or aldosterone group, rats under anesthesia with ketamine 50 mg/kg and xylazine 5 mg/kg given intramuscularly, were implanted subcutaneously a model 2002 mini-osmotic pump (Alza Corporation, Palo Alto, CA, U.S.A.) that infuses 0.5 µL/hr for 6 weeks. The mini-osmotic pumps were replaced every 2 weeks under anesthesia. The mini-osmotic pumps infused subcutaneously 0.75 µg/hr/day aldosterone (Sigma Chemical Co., St. Louis, MO, U.S.A.) dissolved in 0.9% saline or saline alone. Six aldosterone-salt rats received losartan (30 mg/kg per day) to block angiotensin II type I receptor. Losartan was added to the drinking water. Six aldosterone-salt rats were treated with spironolactone (200 mg/kg per day in food). Finally, six aldosterone-salt rats received apocynin (1.5 mM/L) to block activity of NAD(P)H oxidase. Apocynin was added to the drinking water (approximately 300 µM/day). All rats were offered 0.9% saline to drink. Systolic blood pressure (BP) was measured weekly by the tail-cuff method and recorded by a computerized BP monitor (IITT Model 31 NIBP software, IITC Inc. Life Science, Woodland Hills, CA, U.S.A.). Rats were sacrificed at the end of the experiment and heart and kidney wet weights measured. The aorta, heart and kidney were carefully removed, cleaned of excess fat and adventitia, and placed in PSS composed of (mM/L) NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4·7H2O 1.17, NaHCO3 14.9, dextrose 5.5, EDTA 0.26, and CaCl2 1.6.
Aortic homogenate was prepared on ice in lysis buffer containing protease inhibitors (20 mM/L monobasic potassium phosphate (pH 7.4), 1 mM/L EGTA, 10 µg/mL aprotinin, 0.5 µg/mL leupeptin, 0.7 µg/mL pepstatin, and 0.5 mM/L phenylmethylsulfonyl fluoride). Protein content was measured. Activity of NAD(P)H oxidase was measured by lucigenin-enhanced chemiluminescent detection of superoxide in a luminometer (MicroLumatPlus LB 96V, Berthold). The reaction was initiated by the addition of 150 µg of total protein to a 50 mM/L phosphate buffer, pH 7.4, containing 1 mM/L EGTA, 150 mM/L sucrose, 5 µM/L lucigenin as the electron acceptor, and 100 µM/L NADPH as the substrate.
To measure ROS production in frozen cross sections of kidney were stained with dihydroethidium (DHE [10 µM/L]). In the presence of O2-, DHE is converted to the fluorescent molecule ethidium, which can then label nuclei by intercalating with DNA. Fresh-frozen cross sections of kidney (15 µm) were stained with 10 µM DHE (Sigma).
Total RNA was isolated from aorta and kidney using TRIZOL (Invitrogen, Carlsbad, CA, U.S.A.). cDNA was synthesized using Superscript™ III RNase H- reverse transcriptase (Invitrogen) and 5 µg of total RNA primed with oligodT primer. After reverse transcription of RNA into cDNA, real-time polymerase chain reaction (PCR) was performed with Assay-on-Demand™ Gene Expression products consist of a 20× mix of unlabeled PCR primers on Taqman® MGB probe (FAM™ dye-labeled) for the target sequences (p47phox, gp91phox, and p22phox) using the ABI Prism® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, U.S.A.). Housekeeping genes, glyceraldehyde-3-phosphate dehydrogenase, standardized the numbers. Quantification and comparison of mRNA levels between aldosterone infused groups were performed by using the comparative Ct method as described by manufacture.
Small blocks of aorta and kidney from rats were embedded in O.C.T. Tissue-Tek® (Sakura Finetek, Torrance, CA, U.S.A.) and frozen at -70℃. Sections of 5 µm were cut, and immunohistochemistry was performed with standard techniques. Briefly, sections were blocked in 20% horse serum and then incubated overnight (in a humidified box) at 4℃ with a monoclonal antibody against p47phox. For negative control, the primary antibody was replaced with mouse IgG. Biotinylated anti-mouse (DAKO, Carpinteria, CA, U.S.A.) at a dilution of 1:100 in 1.5% horse serum was incubated for 30 min followed by streptavidin conjugated to horseradish peroxidase. Color was developed by the addition of DAB (DAKO). The sections were lightly stained in hematoxylin.
All data analyses were performed with SPSS 10.0 for Windows programs. NAD(P)H oxidase activity and Real-time PCR analyses were performed in duplicate. Results are presented as mean±SD and are compared by ANOVA or by Student t test, where appropriate. The Tukey correction was used to compensate for multiple testing. A value of p<0.05 was considered significant.
The systolic BP of aldosterone-infused rats was significantly higher than that of controls (222±14 vs. 123±9 mm Hg; p<0.01 vs. control). Spironolactone normalized BP to 103±21 mmHg. Aldosterone-mediated elevation of BP was partially decreased by losartan and apocynin during the experiment (170±15 and 189±20 mmHg respectively, at 6 week) (Fig. 1). Body, heart and kidney weight are summarized in Table 1. Aldosterone infusion significantly increased the heart/body weight ratio, which was decreased completely by co-treatment with spironolactone and less so by losartan and apocynin. Similar results were observed in kidneys.
NAD(P)H oxidase activity, a major source of superoxide anion in the vasculature, was increased in aorta of aldosterone-infused rats (75±3 relative light unit [RLU] stimulated by NAD(P)H; p<0.01 vs. control) compared with controls (56±4 RLU). Losartan and spironolactone significantly inhibited NAD(P)H oxidase activity in aorta (53±13 and 44±14.7 RLU) (Fig. 2A). To confirm the effect of NAD(P)H oxidase on O2- generation, dihydroethidium (DHE [10 µM/L]) was compared in kidney. Aldosterone treatment significantly increased the fluorescent signal in the kidney compared with controls. This increase was normalized by treatment with spironolactone and less prominently by treatment with losartan and apocynin (Fig. 2B).
To determine whether increased NAD(P)H-oxidase activity was reflected at the molecular level, real-time PCR was used to compare mRNA expression of the NAD(P)H oxidase subunits, p47phox, p22phox, and gp91phox in aorta and kidney from aldosterone infused rats. Aldosterone-salt rats showed significantly higher p47phox and gp91phox expression in aorta than that of control rats. Spironolactone significantly decreased p47phox and gp91phox mRNA expression. Losartan and apocynin showed a trend of decrease in aorta (Fig. 3A). In kidney, aldosterone-salt rats showed significantly higher p47phox, gp91phox, and p22phox expression than that of control rats. Spironolactone and losartan significantly decreased p47phox, gp91phox, and p22phox mRNA expression. Apocynin showed a trend of decrease in kidney (Fig. 3B).
Finally, we examined the effect of aldosterone infusion on the subcellular distribution of p47phox in aorta and kidney. Immunostaining showed a significant increase in p47phox in the whole aorta of aldosterone-salt rat compared with that of control (Fig. 4A). The aldosterone-induced p47phox overexpression was prevented by spironolactone, and partially by losartan and apocynin. The p47phox expression enhanced in kidney of aldosterone infused rats compared with that of control rats. Spironolactone, losartan, and apocynin inhibited the aldosterone-stimulated p47phox expression in kidney (Fig. 4B).
We previously found that aldosterone infusion into normotensive rats significantly increases blood pressure and induces arterial remodeling (12), cardiac hypertrophy and fibrosis (1), in part through ROS. Our present results demonstrate a critical role of NAD(P)H oxidase and its subunits, p47phox, gp91phox, and p22phox for the vascular oxidant stress response to aldosterone. This activation in NAD(P)H oxidase was abrogated by aldosterone antagonism, and partially reduced by angiotensin II receptor blockade (ARB) and NAD(P)H oxidase inhibition. These results suggest that aldosterone-dependent hypertension and associated target organ damage is mediated by NAD(P)H oxidase-derived ROS. Activation of angiotensin receptor may also contribute to BP elevating effects of aldosterone.
Aldosterone administration in rats increases vascular O2- formation. A specific pro-oxidative effect of aldosterone, mediated through NAD(P)H oxidase was demonstrated in circulating blood cells and in aortic cells (2, 9). The overactivity of NAD(P)H oxidase, a major determinant of vascular tissue redox state, is associated with upregulation of p22phox mRNA, a major subunit of the enzyme, which results in impaired NO-dependent relaxation and media hypertrophy in SHR (13), stroke-prone SHR (SHRsp) (14, 15), DOCA-salt rat (16), angiotensin II-treated rats (17, 18), or aldosterone hypertensive rat (9, 19). Although p22phox alone does not support superoxide production but it has been clearly shown to stabilize the expression of gp91phox (20) and Nox1 (21) in culture, probably serving as a stabilizing and/or regulatory subunit. p22phox overexpression in transgenic (Tg) mice (Tgp22smc) that overexpress the p22phox subunit of NAD(P)H oxidase selectively in smooth muscle, concomitantly upregulates Nox1, and potentates angiotensin II induced vascular hypertrophy (22). And the complete inhibition of p22phox mRNA expression by stable transfection of antisense p22phox cDNA into vascular smooth cells results in a significant inhibition of angiotensin II-stimulated NADH/NADPH-dependent superoxide production, subsequent hydrogen peroxide production, and (3H)leucine incorporation (17). Therefore, p22phox is a critical component of superoxide-generating vascular NADH/NADPH oxidase and suggest a central role for this oxidase system in vascular hypertrophy.
Likewise, p47phox is the protein that carries the cytosolic proteins to the membrane proteins to assemble the active oxidase. The p47phox subunit has been shown to be essential for ROS production in response to phorbol ester (PMA), AngII, thrombin, and platelet-derived growth factor in vascular smooth muscle cells (23, 24) and tumor necrosis factor alpha (TNF-α) and PMA in endothelial cell (25). In the present study, we have shown that aldosterone treatment induced overexpression of two membrane-bound elements (p22phox and gp91phox) and one cytosolic component (p47phox) of the NAD(P)H oxidase and its activity, and led to the translocation of the cytosolic p47phox to the plasma membrane in aorta and kidney. The p22phox may individually interact with cytosolic regulatory elements, p47phox and Rac-1, and therefore, both increased expression of p22phox and p47phox will lead to activation of the enzyme. These findings are consistent with the concept that the translocation of p47phox to membrane is involved in the overall activation of NDA(P)H oxidase, resulting in O2- production in aorta and kidney. Similarly, aldosterone infusion showed elevated renal tissue ROS levels which were associated with increased mRNA expression of p22phox, Nox-4, and gp91phox (10). And in addition to (NAD(P)H) oxidase pathway, the angiotensin-converting enzyme (ACE)/epidermal growth factor receptor (EGFR)/extracellular signal-regulated kinases (ERK) pathway, were known to contribute to the pathogenesis of aldosterone/salt-induced hypertensive rats (26). Even with chronic administration of subdepressor doses of spironolactone (20 mg/kg/day), perivascular fibrosis and coronary microvascular hyperplasia were ameliorated in these models, by blocking ACE/EGFR/ERK pathway, NAD(P)H oxidase/LOX-1 pathway, and Rho-kinase pathway (26). And spironolactone blocked connective tissue growth factor (CTGF) expression which was inducted via p38MAPK by aldosterone in ventricular myocytes (27). This could, at least, explain that anti-aldosterone therapy have cardiovascular protective effects beyond lowering blood pressure.
In the present study, a total blockade of the pro-oxidative effects of aldosterone on aortic segments and kidney was obtained only when the rats were treated with a mineralocorticoid receptor antagonist, spironolactone, which prevented oxidative stress increase, overexpression of NAD(P)H oxidase subunits and BP elevation. The ability of spironolactone to prevent oxidative stress in arterial walls could be linked to the amelioration of target organ damage (28). The underlying mechanism of anti-oxidative blood pressure lowering effects of spironolactone can be explained through its potent inhibitory effects on NAD(P)H oxidase activity. In our experiment and others (29), NAD(P)H oxidase inhibitor, apocynin, attenuated development of hypertension and cardiorenal hypertrophy, with partially decreasing NAD(P)H Oxdase subunits and activity. Yoshida et al. used tempol, a superoxide dismutase mimetic, to examine the possible contribution of superoxide to aldosterone-induced blood pressure elevation and cardiac damage. It blocked blood pressure rise by aldosterone infusion, but failed to inhibit cardiac hypertrophy, inflammatory lesions, and osteopontin gene expressions. Of note, in contrast to apocynin and tempol, N-acetylcysteine, significantly prevented aldosterone-induced cardiac hypertrophy and inflammation, and osteopontin gene expression (30). N-acetylcysteine is a precursor of L-cysteine and a source of sulfhydryl groups in cells and act as a scavenger of free radicals such as hydroxyl radical (OH·) and hydrogen peroxide (H2O2) but not superoxide. Recently, we have shown that the increased vascular oxidative stress in mineralocorticoid-salt model was associated with activation of the endothelin (ET) system via endothelin A receptor pathways (12, 31), showing that endothelin and ROS was identified as important mediators in the pathogenesis of hypertension and associated vascular damage. In the following study, it was found that endothelin stimulates mitochondria to generate ROS, which is independent to activation of eNOS in the vascular wall (32). Therefore, these can explain less effects of apocynin in this model. However, a clear explanation on this mechanism is still not available, and therefore, further studies are needed to elucidate the detailed role of oxidative stress in aldosterone-induced cardiac injury.
ARB was thought to protect against its detrimental effects on target organs, including the cardiovascular system. Although ARB reduces aldosterone levels initially, the suppression of aldosterone is variable and rebound or escape over the long term (33). If not inhibited by ARB, residual aldosterone can cause a wide range of pathophysiologic action and compound the effects of angiotensin II and other products of the RAAS. In some experiment, RAS blocker with either losartan or enalapril did not prevent hypertension development and renal injury in uninephrectomized aldosterone-salt rat (34). Secondly, in our previous experiment, ET-1 was a critical role for the BP elevation and structural alterations of vessels and heart in aldosterone and salt-induced hypertension. Therefore, ARB can't be enough to block the elevated ET-1 in this model (12). Lastly, the usual dose of ARB has no or partial effect on blood pressure or proteinuria and they need ultra-high dose of ARB to ameliorate the progression of aldosterone/salt-dependent renal injury. As shown in this study, losartan partially reduced target organ damage in aldosterone-salt rat.
In conclusion, we have presented strong evidence for the pro-oxidative properties of aldosterone in aorta and kidney in aldosterone-salt rat. Furthermore, we demonstrated that aldosterone activated NAD(P)H oxidase and increased the expression of membrane-bound elements (p22phox and gp91phox) and cytosolic components (p47phox) of the enzyme, which may be linked to cardio-vascular-renal damage. This effect is mediated, at least in part, by the mineralocorticoid receptor, because aldosterone blockade completely prevented BP elevation, increased production of oxidative stress, hypertrophy. Partial beneficial effect of angiotensin II receptor blockade or NAD(P)H oxidase inhibition suggest that co-treatment of aldosterone antagonist with angiotensin receptor or NAD(P)H oxidase blockade may enhance beneficial antioxidative effects and thereby better protect against target organ damage associated with hypertension.
Figures and Tables
 | Fig. 1Systolic BP of aldosterone-infused hypertensive rats was slightly but significantly elevated relative to that of normotensive control rats after 6 weeks of treatment, and showed further elevation thereafter. Administration of spironolactone treatment prevented development of hypertension. Losartan and apocynin showed a trend of decrease.
*, p<0.01 aldosterone vs. aldosterone+losartan or aldosterone+spironolactone or aldosterone+apocynin; †, p<0.01 aldosterone+spironolactone vs. aldosterone+losartan or aldosterone+apocynin (mean±SEM).
|
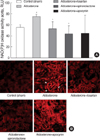 | Fig. 2NAD(P)H oxidase activity is increased in aorta from aldosterone-infused rats. (A) Activity of NAD(P)H oxidase in aortic segments from aldosterone-infused rats were detected by lucigenin chemiluminescence. *, p<0.01 vs. control rats; †, p<0.05 vs. aldosterone-infused rats. (B) Aldosterone-indused superoxide (O2-) production is attenuated in kidney of spironolactone, losartan and apocynin treated rats. O2- levels were determined by fluorescent dihydroethidium (DHE [10 µM/L]) and visualized by confocal microscopy. Arrow indicates intense O2- production.
RLU, relative light units.
|
 | Fig. 3Treatment with spironolactone attenuated NAD(P)H oxidase mRNA expression in aorta (A) and kidney (B) of aldosterone-infused rats. The mRNA expression of the NAD(P)H oxidase subunits p47phox, gp91phox, and p22phox was markedly increased in aldosterone-infused rats. The treatment with spironolactone significantly reduced NAD(P)H oxidase subunits mRNA expression. Losartan and apocynin decreased NAD(P)H oxidase subunits mRNA expression in aorta and kidney from aldosterone-infused rats. *, p<0.05 vs. control rats; †, p< 0.05 vs. aldosterone-infused rats. |
 | Fig. 4Immunostaining for NAD(P)H oxidase subunit, p47phox in aorta (A) and kidney (B) of control rats, aldosterone-salt rat, aldosterone-salt rat treated with losartan, spironolactone and apocynin. (A) The expression of p47phox was increased in aorta of aldosterone-infused rats. Spironolactone significantly decreased p47phox expression in aorta of aldosterone-infused rats. (B) The expression of p47phox was increased in kidney aldosterone-infused rats. Spironolactone significantly decreased p47phox expression in aorta of aldosterone-infused rats. |
References
1. Park JB, Schiffrin EL. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002. 15:164–169.

2. Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004. 109:2213–2220.
3. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999. 341:709–717.
4. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003. 348:1309–1321.

5. Losel R, Schultz A, Boldyreff B, Wehling M. Rapid effects of aldosterone on vascular cells: clinical implications. Steroids. 2004. 69:575–578.

6. Oberleithner H. Aldosterone makes human endothelium stiff and vulnerable. Kidney Int. 2005. 67:1680–1682.

7. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002. 161:1773–1781.
8. Gerling IC, Sun Y, Ahokas RA, Wodi LA, Bhattacharya SK, Warrington KJ, Postlethwaite AE, Weber KT. Aldosteronism: an immunostimulatory state precedes proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003. 285:H813–H821.

9. Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone-infused rats. Biochem Biophys Res Commun. 2004. 313:812–817.

10. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004. 43:841–848.

11. Calò LA, Zaghetto F, Pagnin E, Davis PA, De Mozzi P, Sartorato P, Martire G, Fiore C, Armanini D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004. 89:1973–1976.
12. Park JB, Schiffrin EL. ET(A) receptor antagonist prevents blood pressure elevation and vascular remodeling in aldosterone-infused rats. Hypertension. 2001. 37:1444–1449.
13. Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Etayo JC, Díez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000. 35:1055–1061.

14. Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001. 38:606–611.

15. Park JB, Touyz RM, Chen X, Schiffrin EL. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2002. 15:78–84.

16. Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000. 101:1722–1728.

17. Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996. 271:23317–23321.
18. Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q 4th, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997. 80:45–51.

19. Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001. 38:1107–1111.

20. Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000. 91:21–27.

21. Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med. 2004. 37:1542–1549.

22. Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005. 288:H37–H42.

23. Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001. 104:79–84.

24. Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Invest. 2001. 108:1513–1522.

25. Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002. 90:143–150.
26. Nakano S, Kobayashi N, Yoshida K, Ohno T, Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005. 28:925–936.

27. Lee YS, Kim JA, Kim KL, Jang HS, Kim JM, Lee JY, Shin IS, Lee JS, Suh W, Choi JH, Jeon ES, Byun J, Kim DK. Aldosterone upregulates connective tissue growth factor gene expression via p38 MAPK pathway and mineralocorticoid receptor in ventricular myocytes. J Korean Med Sci. 2004. 19:805–811.

28. Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation. 2005. 111:420–427.

29. Zhang Y, Chan MM, Andrews MC, Mori TA, Croft KD, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not allopurinol prevents and reverses adrenocorticotropic hormone-induced hypertension in the rat. Am J Hypertens. 2005. 18:910–916.

30. Yoshrida K, Kim-Mitsuyama S, Wake R, Izumiya Y, Izumi Y, Yukimura T, Ueda M, Yoshiyama M, Iwao H. Excess aldosterone under normal salt diet induces cardiac hypertrophy and infiltration via oxidative stress. Hypertens Res. 2005. 28:447–455.

31. Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003. 42:811–817.
32. Callera GE, Tostes RC, Yogi A, Montezano AC, Touyz RM. Endothelin-1-induced oxidative stress in DOCA-salt hypertension involves NADPH-oxidase-independent mechanisms. Clin Sci (Lond). 2006. 110:243–253.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download