Abstract
We have evaluated the efficacy and safety of cetuximab plus FOLFIRI for irinotecan and oxaliplatin-refractory colorectal cancers. From September 2004 to February 2006, 31 patients with metastatic colorectal cancer were treated with cetuximab (400 mg/m2 intravenously [IV] over 2 hr on day 1 followed by weekly 1-hr infusions of 250 mg/m2) plus bi-weekly FOLFIRI (irinotecan 150 mg/m2 IV over 90 min, and leucovorin 100 mg/m2 IV over 2 hr, followed by 5-FU 400 mg/m2 IV bolus on day 1, and followed by 5-FU 2,400 mg/m2 by continuous IV over 46 hrs). Patients received a median of four cycles (range: 1-23). Eight (25.8%) patients had confirmed partial responses and 10 (32.2%) had stable disease. After a median follow-up of 13.2 months for surviving patients, the median time to progression was 2.9 months, the median duration of response was 5.4 months, and the median overall survival was 10.9 months. Skin toxicity was observed in 25 patients (80.4%) including grade 3 in 6 patients (19.4%). Other common non-hematologic toxicities of all grades were mucositis (32.3%), asthenia (22.6%), diarrhea (12.9%), and paronychial cracking (12.9%). The combination of cetuximab with FOLFIRI was effective and tolerable in colorectal cancer patients heavily pretreated with a number of chemotherapy regimens.
Colorectal cancer is the fourth most common malignancy in Korea, with 10,000 new cases and 5,000 deaths each year, and its incidence is increasing (1). About 20% of colorectal cancer patients are diagnosed in the metastatic stage and, even if curative surgical treatment is performed, about 40% of patients will experience local or distant recurrences (2).
Until the early 1990s, 5-fluorouracil (5-FU) was considered the only effective chemotherapeutic drug for colorectal cancer. In the past decade, however, the introduction of the topoisomerase-I inhibitor irinotecan and the third-generation platinum derivative oxaliplatin has increased the median survival among patients with advanced colorectal cancer (3, 4). Currently, irinotecan and oxaliplatin are used in combination with 5-FU and leucovorin as first and second-line treatment for advanced colorectal cancer.
The epidermal growth factor receptor (EGFR) is a member of the HER family of receptors, which are involved in signaling pathways affecting cell growth, differentiation, proliferation and programmed cell death. The receptors are overexpressed in many solid tumors, and the overexpression of EGFR has been associated with poor prognosis in patients with colorectal cancer (5). Clinically, this receptor can be inhibited by the monoclonal antibody cetuximab or by the tyrosine kinase inhibitors gefitinib and erlotinib.
Cetuximab is a monoclonal antibody directed against the extracellular binding domain of the EGFR. In advanced colorectal cancer patients refractory to irinotecan alone or to combination chemotherapy including irinotecan, the combination of cetuximab and irinotecan showed improved response rates and an increased median time to disease progression compared with cetuximab alone (6).
We therefore assessed the efficacy and safety of a combination of weekly cetuximab and biweekly irinotecan and infusional 5-FU/leucovorin (FOLFIRI) and the association between the EGFR status and response.
From September 2004 to February 2006, patients who fulfilled the following eligibility criteria were enrolled in this phase II study. The eligibility criteria for this study were 1) histologically confirmed metastatic colorectal adenocarcinoma without central nervous system (CNS) metastasis, 2) prior palliative chemotherapy with fluoropyrimidine (5-FU, capecitabine), irinotecan and oxaliplatin, 3) documented progression of radiological evidence during chemotherapy with these agents, 4) at least one unidimensionally measurable lesion according to Response Evaluation Criteria In Solid Tumors (RECIST), 5) performance score (PS) of 0-2 according to the Eastern Cooperative Oncology Group (ECOG) scale, and 6) adequate bone marrow function (hemoglobin ≥10.0 g/dL; leukocyte ≥4,000 cells/µL; platelet ≥100,000 cells/µL), adequate liver function (serum bilirubin level ≤2.0 mg/dL; serum transaminase level ≤3 times the upper limit of the normal range) and renal function (serum creatinine level ≤1.4 mg/dL).
Immunohistochemical evidence of the EGFR expression was measured semiquantitatively (>0 on a scale of 0, 1+, 2+, or 3+) in a single reference laboratory. These measurements were performed at the Central Laboratory (Prince of Wales Hospital, Sha Tin, Hong Kong) and graded using a commercially available kit (EGFRpharmDx; Dako Corporation, Carpinteria, CA, U.S.A.) on paraffin-embedded tumor specimens according to the manufacturer's instructions.
This study was designed as a phase II trial. All patients provided written informed consent, and the institutional review boards (IRB) of Asan Medical Center approved the study protocol. In this study, the estimation of the sample size was as below. The Simon two-stage phase II design provided 90% power and a 0.05 level of significance overall to distinguish between the null and alternative hypotheses, where the null hypothesis (H0) is that the true overall response rate is <5%, and the alternative hypothesis (H1) is that the true overall response rate is >25%. The trial would be terminated at nine patients if zero patients have responded or be studied at 30 patients if the trial goes on to the second stage.
Cetuximab (400 mg/m2) was administered intravenously (IV) on day 1 over 2 hr, followed by weekly 1-hr infusions of 250 mg/m2. A histamine-receptor antagonist was given as premedication before at least the first infusion. FOLFIRI consisted of irinotecan (150 mg/m2) IV over 90 min, and leucovorin (100 mg/m2) IV over 2 hr, immediately followed by 5-FU (400 mg/m2) IV bolus on day 1, and followed by 5-FU (2,400 mg/m2) by continuous IV over 46 hr. Patients were scheduled to receive biweekly FOLFIRI with weekly cetuximab. If a patient experiences grade 3 of skin toxicity, cetuximab therapy may be deferred for up to two consecutive weeks without changing the dose level. If the toxicity resolves to grade 2 or less by the following treatment period, the treatment may resume. With the second or third occurrences of grade 3 skin toxicity, cetuximab therapy may be deferred again for up to two consecutive weeks with concomitant dose reductions to 200 or 150 mg/m2, respectively. Patients had to discontinue cetuximab if more than two consecutive infusions were withheld or a fourth occurrence of grade 3 skin toxicity occurred despite an appropriate dose reduction. Cetuximab was not withheld for FOLFIRI-related toxicity. The dose of FOLFIRI was reduced by 25% if grade 3 or higher non-hematologic toxicities (such as stomatitis or diarrhea) were observed during the treatment period or grade 2 hematologic toxicity (leukocyte 3,000-4,000 cells/µL or platelet 75,000-100,000 cells/µL) was observed immediately prior to administration of the FOLFIRI regimen. If there was grade 3 or higher leukopenia or thrombocytopenia, the FOLFIRI regimen was delayed until recovery.
The primary end point was the rate of confirmed radiologic tumor response, according to RECIST after every three cycles of chemotherapy. Radiologic evaluation consisted of chest radiography and abdominopelvis CT scans. Secondary end-points were the evaluation of time to disease progression (TTP), time to treatment failure (TTF), overall survival (OS) and the safety profile in the intent-to treat analysis. TTP was defined as the time from treatment start to either progression of cancer or death from any cause. TTF was defined as the time from treatment start to either discontinuation of treatment or death from any cause. OS was defined as the time from treatment start to death from any cause. Survival curves were estimated using the Kaplan-Meier method. Safety was assessed in terms of toxicity and evaluated as grades 1 to 4 based on the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
To assess the relative significance of potential prognostic factors, we performed univariate and multivariate analyses using the log-rank test and the Cox proportional hazards models, respectively. A p value <0.05 was considered statistically significant, and all analyses were performed using SPSS 12.0 for Windows.
From September 2004 to February 2006, a total of 31 patients met the eligibility criteria; their baseline characteristics are listed in Table 1. Of these patients, 25 (80.6%) underwent surgical resection of their primary tumor and 14 (45.2%) had received more than 2 regimens of palliative chemotherapy. The median number of cycles of cetuximab plus FOLFIRI administered was four (range: 1-23).
The overall response rate (i.e. complete responses [CR]+partial responses [PR] rates) was 25.8% (95% CI, 10.4-41.2%). The median duration of response was 5.4 months (95% CI, 2.1-8.7 months). The disease control rate (i.e. CR+PR+stable disease [SD]) was 58.0% patients (95% CI, 40.6-75.4%) (Table 2).
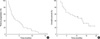
Of the 31 patients, 11 (33.3%) remained alive at a median follow-up of 13.2 months. The median TTP was 2.9 months (95% CI, 1.4-4.4 months) and the median TTF was 2.1 months. Treatment failure was caused by disease progression (87.0 %), financial burden (6.5%), and inability to tolerate treatment (6.5%). The median OS was 10.9 months (95% CI, 3.8-18.0 months), and the 1-yr OS rate was 47.6% (Fig. 1).
Among the 15 patients whose tumor tissue was available to test for EGFR expression, 13 (86.7%) had tumor cell expression ranging from 1+ to 3+. The presence or degree of EGFR expression did not correlate significantly with clinical response rate (p=0.32) (Table 3).
The 31 patients received 212 cycles of chemotherapy. Safety evaluation showed that the most common hematologic toxicity was neutropenia (54.8%), followed by thrombocytopenia (3.2%). Grade 3 or higher neutropenia developed in 11 (35.5%) patients, but there were no incidents of neutropenic fever or treatment-related mortality. An acne-like skin rash was observed in 25 (80.6%) patients, with grade 3 toxicity in 6 (19.4%). After the sixth administration of cetuximab (median two, range 1-6), almost all patients developed a skin rash. Other common non-hematologic toxicities were mucositis (32.3%), asthenia (22.6%), diarrhea (12.9%), and paronychial cracking (12.9%) (Table 4).
There was a correlation between the presence and severity of the acne-like skin toxicity and response rate and survival. As shown in Table 5, there were superior response rates (p=0.02) and survival rates (p<0.01) with higher grades of skin toxicity.
Univariate analysis of the relationship between survival outcome and clinicopathologic factors showed that the absence of skin rash was significantly associated with TTP, whereas poor performance status and the absence of skin rash were significant negative prognostic factors for OS. Multivariate analysis also identified the absence of skin rash as an independent factor indicative of poor prognosis for TTP, and the poor performance status and the absence of skin rash were independent prognostic factors negatively affecting the overall survival (Table 6).
We have evaluated the efficacy, safety and clinical feasibility of cetuximab plus FOLFIRI for patients with irinotecan and oxaliplatin-refractory advanced colorectal cancer. The EGFR-targeting monoclonal antibody, cetuximab, has been shown to be effective in patients with irinotecan-refractory colorectal cancer (6). That study showed that the combination of cetuximab and irinotecan resulted in greater efficacy than cetuximab alone, with a higher objective response rate (22.9% vs. 10.8%), overall disease control rate (55.5% vs. 32.4%), and a prolonged time to progression (4.1 vs. 1.5 months). Our response rate was comparable with the result of that study. In addition, our results confirm that pretreatment with oxaliplatin did not have a negative impact on the response to cetuximab plus irinotecan since all our patients had been previously treated with oxaliplatin.
Although the pivotal studies combined cetuximab with irinotecan, the current study involves 5-FU and leucovorin in addition. As cetuximab potentiates the anti-tumor effects of irinotecan significantly compared with either irinotecan or cetuximab monotherapy in vivo and in vitro (7), the same or similar synergistic effect is expected in combination of 5-FU and cetuximab. In addition, the result of irinotecan plus 5-FU/LV was superior to that of irinotecan monotherapy for 5-FU-refractory colorectal cancer (3). A recent study showed that the pharmacokinetic properties of cetuximab were unaffected by co-administration of 5-FU in comparison with administration of cetuximab as a single agent or cetuximab plus irinotecan (8). Our study showed comparable side effects with another study in which salvage FOLFIRI regimen was given to patients with refractory advanced gastric cancer except skin toxicity, a typical side effect of EGFR targeted therapies (9). Therefore, the addition of 5-FU/leucovorin to irinotecan and cetuximab is considered to be effective and tolerable to patients who were heavily pretreated with many chemotherapy regimens. A comparative study to evaluate the efficacy and safety of the FOLFIRI regimen versus FOLFIRI plus cetuximab is underway.
Acne-like skin toxicity, an adverse event typical of cetuximab as well as of other anti-EGFR agents, was associated with increased response rates and a prolonged median survival time in patients with colorectal cancer. Furthermore, in agreement with previous studies we found that higher-grade skin toxicities were associated with a longer TTP (10, 11). Although the mechanism of correlation between skin toxicity and tumor response has not been fully clarified, skin toxicity is currently regarded as a surrogate marker of tumor response as has been shown by our analysis of prognostic factors. As in previous studies we could also show that tumor expression of EGFR did not correlate with objective tumor responses (6, 12). Thus, the EGFR expression measured by the current test-kit does not seem to predict the treatment outcome. Therefore, in clinical practice, no patient should be included or excluded from cetuximab therapy on the basis of EGFR test results (13). Factors that can predict antitumor response or clinical benefit from cetuximab therapy are currently under investigation (14, 15), both to optimize therapeutic indications and to reduce toxicities and costs.
More recently, clinical trials incorporated cetuximab into first-line (8) or adjuvant (16) treatment settings for metastatic colon cancer and also evaluated the use of cetuximab with other targeted agents (17, 18), such as bevacizumab, gefitinib and erlotinib. In addition, cetuximab with oxaliplatin-based chemotherapy (5-FU, leucovorin, oxaliplatin: FOLFOX) has been reported to show similar efficacy data compared to cetuximab when it is combined with FOLFIRI (19). Its effectiveness in colorectal and head-and-neck cancers (20) has prompted the investigation of cetuximab in the treatment of other solid tumors.
Although patients with advanced cancer have a greater opportunity to receive these treatments, the high cost of the treatments can lead to a considerable financial burden. For example, an eight-week course of the FOLFIRI plus cetuximab regimen costs nearly $30,790 in the United States and about $23,600 in Korea (21). Cost is an important factor determining which patients with advanced-stage cancer to receive treatment. In fact, the financial burdens led to treatment discontinuance in 6.5% of our patients and even discouraged patients who wanted to receive cetuximab.
Despite a limitation of the small sample size, we have shown here that the combination of weekly cetuximab and biweekly FOLFIRI is effective as third-line treatment in irinotecan and oxaliplatin-refractory colorectal cancer patients. This treatment regimen was also safe and tolerable, but its cost-effectiveness still needs to be evaluated.
Figures and Tables
ACKNOWLEDGMENT
Immunohistochemical staining of tumor tissue (at the Central Laboratory, Prince of Wales Hospital, Sha Tin, Hong Kong) was supported by Merck Korea.
References
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.

2. Desch CE, Benson AB 3rd, Smith TJ, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Somerfield MR. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999. 17:1312.

3. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000. 343:905–914.

4. Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004. 22:23–30.

5. Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993. 71:2454–2460.
6. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004. 351:337–345.

7. Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002. 8:994–1003.
8. Folprecht G, Lutz MP, Schoffski P, Seufferlein T, Nolting A, Pollert P, Kohne CH. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006. 17:450–456.

9. Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, Park YS, Im YH, Park K. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005. 92:1850–1854.

10. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004. 22:1201–1208.

11. Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, Tonini G. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. 2006. 94:792–797.

12. Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005. 23:1803–1810.

13. The NCCN Clinical Practice Guidelines in Oncology: Guidelines for Treatment of Cancer by Site, Colon/Rectal Cancer. 2006. accessed 29 September 2006. Available at http://nccn.org/professionals/physician_gls/PDF/colon.pdf
.
14. Shia J, Klimstra DS, Li AR, Qin J, Saltz L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, Gerald W, Chen B. Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Mod Pathol. 2005. 18:1350–1356.

15. Vallbohmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, Rhodes KE, Sherrod AE, Iqbal S, Danenberg KD, Groshen S, Lenz H-J. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005. 23:3536–3544.
16. Alberts SR, Sinicrope FA, Grothey A. N0147: a randomized phase III trial of oxaliplatin plus 5-fluorouracil/leucovorin with or without cetuximab after curative resection of stage III colon cancer. Clin Colorectal Cancer. 2005. 5:211–213.

17. Meyerhardt JA, Heseltine D, Ogino S, Clark JW, Enzinger PC, Ryan DP, Earle CC, Zhu AX, Fuchs CS. Efficacy of cetuximab after treatment with oral epidermal growth factor receptor tyrosine kinase inhibitor-based chemotherapy in metastatic colorectal cancer. Clin Colorectal Cancer. 2006. 6:59–65.

18. Cetuximab and/or Bevacizumab Combined With Combination Chemotherapy in Treating Patients With Metastatic Colorectal Cancer. National Cancer Institute (NCI). 2006. accessed 29 September 2006. Available at
http://ClinicalTrials.gov
.
19. Venook A, Niedzwiecki D, Hollis D, Sutherland S, Goldberg R, Alberts S, Benson A, Wade J, Schilsky R, Mayer R. Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) ± cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results. J Clin Oncol. 2006. 24:3509.

20. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006. 354:567–578.

21. Schrag D. The price tag on progress-chemotherapy for colorectal cancer. N Engl J Med. 2004. 351:317–319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download