Abstract
To investigate the characteristics of incidental pituitary microadenomas, we examined 120 pituitary glands from Korean forensic autopsy cases, from which eight tumors were identified (incidence 6.7%). The average age of the affected subjects was 50 yr (range: 33-96 yr) with a female predominance. The maximum diameters of the tumors ranged from 0.4 to 5.4 mm (mean: 2.8 mm). Immunohistochemical analysis of pituitary hormones revealed three growth hormone-secreting adenomas, one prolactin-producing adenoma, one gonadotropin-producing adenoma, one plurihormonal adenoma, and two null cell adenomas. MIB-1 staining for Ki-67 antigen showed no positive expression. The microvessel density (MVD) of the pituitary microadenomas ranged from 2.3 to 11.6% (mean: 5.3%) and was significantly lower than that of nonneoplastic pituitary glands (11.9-20.1%, mean: 14.8%). Our study provides reference data on incidental pituitary microadenomas in the Korean population.
With technical advances in radiology, incidental pituitary microadenomas (adenomas less than 10 mm in diameter) are detected more frequently nowadays. The clinical approach and treatment of these lesions, however, are controversial because the majority of lesions are not associated with clinical signs or obvious abnormalities during the subject's lifetime (1-4). Since the first report of 2 cases of pituitary microadenomas without clinical manifestations in 1903, several studies have investigated the incidence and natural history of these tiny tumors (5-8). However, no previous study of incidental pituitary microadenomas has been conducted in Korea. To determine the characteristics of incidental pituitary microadenomas in the Korean population, we investigated pituitary glands obtained from Korean forensic autopsy cases.
Five hundred and eighty-five forensic autopsies were performed at the Department of Forensic Medicine, Central District of the National Institute of Scientific Investigation, Daejeon, Korea, between March 2003 and February 2004. From these cases, only 120 pituitary glands were available for histologic sections because of putrefaction or organ damage from various causes, the subjects being foreigners, or for other reasons. Each gland was fixed in 10% formalin and cut in the sagittal plane. Two to five slices (average: four slices) of the pituitary gland were prepared at 1 to 2 mm intervals, embedded in paraffin, processed using the standard method, and stained with hematoxylin and eosin (H&E).
The H&E-stained slides were reviewed, and the diagnosis of adenoma was made based on the following histologic criteria: a circumscribed group of cells exhibiting cellular uniformity, loss of the normal acinar and stromal patterns, and compression of the adjacent pituitary parenchyma. The pituitary glands that met these criteria were then stained for reticulin to confirm the loss of the stromal pattern and compression of the adjacent pituitary gland.
The adenomas were stained immunohistochemically using primary antibodies against the pituitary hormones, prolactin (PRL) (1:100, Novocastra, Newcastle, U.K.), growth hormone (GH) (1:100, Novocastra), adrenocorticotropic hormone (ACTH) (1:300, DAKO, Glostrup, Denmark), thyroid-stimulating hormone (TSH) (1:100, Novocastra), luteinizing hormone (LH) (1:500, NeoMarkers, Fremont, CA, U.S.A.), and follicle-stimulating hormone (FSH) (1:100, Immunon, Pittsburgh, PA, U.S.A.). To determine the proliferation index, an antibody against MIB-1 (1:100, Zymed, San Francisco, CA, U.S.A.) was used. An antibody against CD34 (1:100, Novocastra) was applied to determine the microvessel density (MVD) using a Lab Vision Autostainer™ (Lab Vision Co., Fremont, CA, U.S.A.) according to the manufacturer's instructions. Diaminobenzidine was used as a chromogen.
The Ki-67 antigen labeling index (LI) with the MIB-1 antibody was determined manually and expressed as the percentage of positive nuclei in at least five fields chosen randomly at ×400 magnification.
MVD was examined semiautomatically with CD34-immunostained sections using a computer image analysis system (Image Pro Plus; Media Cybernetics, Silver Spring, MD, U.S.A.). The MVD was calculated by measuring the mean percentage of the area occupied by vessels in digitally captured images of microadenomas and nonneoplastic parenchyma under a microscope (BX51; Olympus, Tokyo, Japan) with a digital camera (DMC2; Polaroid, Cambridge, MA, U.S.A.). In each case, images were taken from three different areas at ×200 magnification. The paired t-test was used to determine the difference in the MVD between microadenomas and non-neoplastic parenchyma using the SPSS software package (SPSS Inc., Chicago, IL, U.S.A.).
Of the 120 pituitary glands, 61 glands were from males and 59 were from females. The age distribution ranged from neonates to 96 yr (0 to 10 yr, n=13; 11 to 20 yr, n=11; 21 to 30 yr, n=22; 31 to 40 yr, n=19; 41 to 50 yr, n=20; 51 to 60 yr, n=13; 61 to 70 yr, n=16; 71 to 96 yr, n=6). The cause of death varied and included natural diseases such as ischemic heart disease and unnatural causes such as hanging, drowning, and multiple injuries. Two cases had diabetes, while the remainder had no medical history of endocrine disorders. Nine lesions in eight pituitary glands were suspected of being microadenomas based on H&E staining (Fig. 1A), and eight microadenomas were confirmed in seven pituitary glands with reticulin stain (Fig. 1B) (incidence 6.7%). One case was hyperplasia, six cases were single microadenomas, and the remaining two pituitary microadenomas were contained in one gland. Five of them were in females and three were in two males. Their ages ranged from 33 to 96 yr and averaged 50 yr. The maximum diameter of the tumors ranged from 0.4 to 5.4 mm (mean: 2.8 mm).
Three of the eight microadenomas were diffusely positive for GH (37.5%), one was diffusely positive for PRL (12.5%), one was diffusely positive for PRL, GH, and TSH (12.5%), and one was diffusely positive for FSH and focally positive for LH (12.5%). The two adenomas found in one pituitary gland (Case 5) showed different immunoreactivities: one was diffusely positive for GH and the other was diffusely positive for PRL, GH, and TSH (Table 1, Fig. 1C). The remaining two adenomas were negative for all hormones (25.0%). The Ki-67 LI could not be determined because no microadenomas expressed MIB-1.
Pituitary microadenomas were detected using computed tomography (CT) or magnetic resonance imaging (MRI) scan in between 3.7% and 37.0% of cases (2, 9), while autopsy studies revealed a prevalence ranging from 1.4 to 27.0% (6, 8). This wide variation might be due to the histological criteria and sectioning methods. Some earlier studies used cellular uniformity and loss of the normal stromal pattern as the only criteria for microadenoma, resulting in a relatively high incidence of pituitary microadenoma (10, 11). Conversely, the reticulin stains, which we used in this study, have rarely been used to confirm the diagnosis of pituitary microadenomas (6, 12). Although the number and thickness of tissue sections might result in different percentages of incidental microadenomas (8, 10), their correlation remains controversial (6, 7, 12). It was reported that there was no relationship between the size of the series involved and the number of adenomas detected (6). In our study, the incidence of pituitary microadenomas (6.7%) was relatively low. One possible explanation might be the larger proportion of younger subjects in our series. Those series that included children in their patient group reported relatively low incidences of pituitary microadenomas, which could be due to the low prevalence of pituitary adenomas in the pediatric population (7, 13, 14). In our study, the incidence of pituitary microadenomas was higher (10.8%) when the subjects under 30 yr of age were excluded. The female predominance in our study differs from previous autopsy studies that showed no sex predominance (6, 8, 12) or a slight male predominance (7). However, clinical studies based on radiology, laboratory findings, and surgery show a slight female predominance, similar to our results (3, 9, 15).
In our study, the two most common types of adenoma were GH-positive adenoma (37.5%) and null cell adenoma (25.0%). Immunohistochemical staining for prolactin was positive in two microadenomas, and only one adenoma was a prolactinoma (12.5%). These results are inconsistent with previous studies in which the reported frequency of prolactinoma was as high as 53.0% (6-8, 13, 16). Interestingly, one microadenoma from a very old woman (Case No. 7) was FSH-positive with focal scattered LH positivity. It is quite possible that the age-related decline in sex hormone levels and subsequent stimulation of gonadotrophs are related to the development of pituitary tumors (13).
The Ki-67 LI with MIB-1 antibody for pituitary adenoma is relatively low compared to other brain tumors, and a Ki-67 LI greater than 3.0% has been suggested as a useful marker of aggressive behaviors, such as recurrence and invasive growth (17). Few studies have determined the Ki-67 LI of pituitary microadenomas even with surgical specimens, and the reported LI of microadenomas ranges from 1.1 to 1.8% (18, 19). A correlation between the Ki-67 LI and the maximum tumor diameter has been suggested, and certain types of microadenoma have a high proliferation index (20). No MIB-1 expression was found in our study, perhaps because the tissues were obtained at autopsy, not from surgical specimens. Nevertheless, possible technical errors during immunohistochemical staining cannot be ruled out, because repeated examination was not possible due to tumor loss after using several sections for immunohistochemical and reticulin staining.
Angiogenesis characterized by a high MVD is essential for tumor growth and metastasis. In contrast to the majority of solid tumors, pituitary adenomas show decreased expression of vascular endothelial growth factor and have significantly lower microvessel densities compared to non-neoplastic pituitary glands (18, 21). Our results are in good agreement with these studies, and suggest that angiogenic inhibitors may play a role in the behavior of these tumors (21, 22). This difference in MVD between adenoma and non-neoplastic pituitary gland could be a useful method to discriminate microadenomas from non-neoplastic pituitary gland in surgical specimens. In our study, a variation in the MVD according to the type of pituitary adenoma was noted. Similar to previous studies (21, 22), prolactinomas had a relatively lower MVD than other types of adenoma. Although a significantly lower vascularity of microprolactinomas has been suggested than that of macroprolactinomas (22), no significant difference in MVD was observed between microadenomas and macroadenomas in adult patients (24).
In conclusion, our study determined the incidence and characteristics of incidental pituitary microadenomas in autopsy cases from the Korean population. Although our study examined a Korean forensic population, it nevertheless provides a valuable reference on incidental pituitary microadenomas in the general Korean population.
Figures and Tables
Fig. 1
(A) Pituitary microadenoma (H&E stain, ×100). (B) Loss of the normal acinar and stromal patterns and compression of the adjacent pituitary parenchyma (reticulin stain, ×100). (C) Positive immunoreactivity with prolactin (×100).
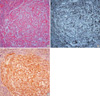
Fig. 3
Microvessel density in microadenomas and non-neoplastic pituitary gland showing a significant difference.

Table 1
The characteristics of incidental pituitary microadenomas

PRL, prolactin; GH, growth hormone; ACTH, adrenocorticotropic hormone; TSH, thyroid-stimulating hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; MVD, microvessel density; MA, microadenoma; NP, non-neoplastic pituitary gland; HCM, hypertrophic cardiomyopathy; SCD, sudden cardiac death; IHD, ischemic heart disease.
References
1. Krikorian A, Aron D. Evaluation and management of pituitary incidentalomas--revisiting an acquaintance. Nat Clin Pract Endocrinol Metab. 2006. 2:138–145.

3. Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol. 2003. 149:123–127.

5. Arita K, Tominaga A, Sugiyama K, Eguchi K, Iida K, Sumida M, Migita K, Kurisu K. Natural course of incidentally found nonfunctioning pituitary adenoma, with special reference to pituitary apoplexy during follow-up examination. J Neurosurg. 2006. 104:884–891.

7. Parent AD, Brown B, Smith EE. Incidental pituitary adenomas: a retrospective study. Surgery. 1982. 92:880–883.
8. Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med. 1981. 304:156–158.

9. Fainstein Day P, Guitelman M, Artese R, Fiszledjer L, Chervin A, Vitale NM, Stalldecker G, De Miguel V, Cornalo D, Alfieri A, Susana M, Gil M. Retrospective multicentric study of pituitary incidentalomas. Pituitary. 2004. 7:145–148.

10. Gorczyca W, Hardy J. Microadenomas of the human pituitary and their vascularization. Neurosurgery. 1988. 22:1–6.

12. Tomita T, Gates E. Pituitary adenomas and granular cell tumors. Incidence, cell type, and location of tumor in 100 pituitary glands at autopsy. Am J Clin Pathol. 1999. 111:817–825.

13. Kastelan D, Korsic M. High prevalence rate of pituitary incidentaloma: Is it associated with the age-related decline of the sex hormones levels? Med Hypotheses. 2007. 69:307–309.

14. Char G, Persaud V. Asymptomatic microadenomas of the pituitary gland in an unselected autopsy series. West Indian Med J. 1986. 35:275–279.
15. Feldkamp J, Santen R, Harms E, Aulich A, Modder U, Scherbaum WA. Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas-results of a prospective study. Clin Endocrinol (Oxf). 1999. 51:109–113.
16. DeStephano DB, Lloyd RV, Pike AM, Wilson BS. Pituitary adenomas. An immunohistochemical study of hormone production and chromogranin localization. Am J Pathol. 1984. 116:464–472.
17. Paek KI, Kim SH, Song SH, Choi SW, Koh HS, Youm JY, Kim Y. Clinical significance of Ki-67 labeling index in pituitary macroadenoma. J Korean Med Sci. 2005. 20:489–494.

18. Vidal S, Kovacs K, Horvath E, Scheithauer BW, Kuroki T, Lloyd RV. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 2001. 438:595–602.

19. Yonezawa K, Tamaki N, Kokunai T. Clinical features and growth fractions of pituitary adenomas. Surg Neurol. 1997. 48:494–500.

20. Losa M, Barzaghi RL, Mortini P, Franzin A, Mangili F, Terreni MR, Giovanelli M. Determination of the proliferation and apoptotic index in adrenocorticotropin-secreting pituitary tumors: comparison between micro- and macroadenomas. Am J Pathol. 2000. 156:245–251.
21. Niveiro M, Aranda FI, Peiro G, Alenda C, Pico A. Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum Pathol. 2005. 36:1090–1095.

22. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab. 2000. 85:1159–1162.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download