Abstract
The growth inhibitory effects of four retinoic acid (RA) derivatives, 9-cis RA, 13-cis RA, N-(4-hydroxyphenyl) retinamide (4-HPR), and all-trans retinoic acid (ATRA) were compared. In addition, the effects of various combinations of these four agents were examined on non-small cell lung carcinoma (NSCLC) cell-lines, and on the expressions of retinoic acid receptors (RARs) and retinoid X receptors (RXRs) on these cells. At the clinically achievable concentration of 1 µM, only 4-HPR inhibited the growths of H1299 and H460 cells-lines. However, retinoic acid receptor β (RARβ) expression was up-regulated on H460 and H1299 cells treated with 1 µM of ATRA, 13-cis RA, or 9-cis RA. All NSCLC cell lines showed growth inhibition when exposed sequentially to 1 µM ATRA and 0.1 µM 4-HPR. In particular, sequential treatment with 1 µM ATRA or 13-cis RA and 4-HPR markedly inhibited H1703 cell growth; these cells exhibited no basal RARβ expression and were refractory to 4-HPR. However, in NSCLC cell lines that expressed RARβ, the expressional levels of RARβ were up-regulated by ATRA alone and by sequential treatment with ATRA and 4-HPR. 4-HPR was found to be the most active of the four agents in terms of NSCLC growth-inhibition. Moreover, sequential treatments with ATRA or 13-cis RA followed by 4-HPR were found to have synergistic growth-inhibitory effects and to regulate RAR expression.
The retinoids, a group of vitamin A derivatives, have been identified as potential cancer treatments and have been reported to exert anti-cancer effects, which include the apoptosis and differentiation of cancer cells (1, 2). The major metabolic forms of vitamin A in vivo are retinol, retinal, and all-trans retinoic acid (ATRA). The 9-cis retinoic acid (RA) and 13-cis RA are natural ATRA isomers, whereas 4-hydroxyphenyl retinamide (4-HPR) is a conformationally restricted synthetic retinoid (3, 4). It has been established that ATRA inhibits the proliferation and induces markers of apoptosis and squamous differentiation in non-small cell lung carcinoma (NSCLC) cell lines (5). A phase II study of ATRA as a single agent in NSCLC showed minimal activity (8% response rate in 28 patients) and mild toxicities (6). Moreover, although 13-cis RA is the best-studied chemopreventive retinoid in the aerodigestive tract (7), 13-cis RA treatment did not reduce the overall rates of second primary tumor development, recurrence, or mortality in stage I NSCLC (8). 9-cis RA is a naturally occurring ligand that exhibits a high degree of affinity for retinoic acid receptors (RARs) and also binds to retinoid X receptors (RXRs) (9). 9-cis RA has been observed to have a chemopreventive effect in an A/J mouse lung carcinogenesis model, and this was accompanied by retinoic acid receptor β (RARβ) upregulation (10). Kurie et al. assessed the efficacies of two retinoid-based regimens, 9-cis RA and 13-β RA+α-tocopherol, in former smokers, since the loss of RARβ expression in bronchial epithelium is considered to be a biomarker of preneoplasia, and 9-cis RA has been reported to increase RARβ expression (11). 4-HPR (Fenretinide) is a synthetic retinoid which inhibits the growths on a large number of lung cancer cell lines, including cell lines unresponsive to ATRA (12). However, no comparative study has been conducted to determine which retinoid is most effective at causing the growth inhibition of NSCLC cells.
4-HPR acts mainly via retinoic acid receptor γ (RARγ), and in part via RARβ (13). On the other hand, ATRA, 13-cis RA, and 9-cis RA are compatible with all RARs/RXRs subtypes (14). The major difference between 4-HPR and the other RA derivatives is the ability to induce apoptosis; however, it does not induce cell differentiation (12). 4-HPR has been shown to induce apoptosis in a variety of epithelial and hematologic malignancies, but the mechanism involved has yet to be clearly elucidated (15, 16). Two possible mechanisms have been proposed to explain 4-HPR-induced apoptosis. The first involves the participation of retinoid receptors in 4HPR-induced cellular events. This suggestion is supported by the observations that 4-HPR-induces RARβ expression in various human carcinoma cell lines (13), and that 4-HPR induces the activations of retinoic acid receptor α (RARα), RARβ, and RXRs (4). The second mechanism involves the interruption of mitochondrial membrane potentials associated with the generation of reactive oxygen species (ROS) by 4-HPR (17). Moreover, 4-HPR appears to be more robust than ATRA with regard to its apoptosis-inducing abilities in NSCLC cells (18). However, the possible clinical use of 4-HPR raises questions concerning the need for long-term treatment and the safe and effective dosage required for the long-term inhibition of cancer cell growth. Thus, novel treatment methods are required with improved clinical efficacies. In the present study, we assessed the growth inhibitory effects of the four RA derivatives, 9-cis RA, 13-cis RA, 4-HPR, and ATRA, when treated singly, sequentially, and simultaneously and compared these treatments with regard to RARβ mRNA expressional induction in NSCLC cell lines. The other objective of this study was to determine whether 4-HPR induces apoptosis by enhancing ROS production or by upregulating retinoid receptor expression. Thus, we conjectured, if 4-HPR induces apoptosis primarily via ROS generation, other retinoids, which act as antioxidants, may exhibit an antagonistic effect when combined with 4-HPR. On the other hand, if 4-HPR acts on RARs, combinations of 4-HPR and other retinoids might result in synergism. In this study, we show that sequential treatments with ATRA or 13-cis RA and 4-HPR can synergistically inhibit NSCLC cell growth via RAR regulation. From the clinical point of view, although in vitro studies have demonstrated that 4-HPR (at concentrations ranging from 1 to 10 µM) suppresses the growths of malignant cells of various histotypes, including NSCLC, the mean serum level of 4-HPR in patients receiving 200 mg daily was only 0.26 µM (19). Even though a higher plasma peak level of 4-HPR could be achieved at higher dosages, plasma peak concentrations of <3 µM appear to be safe (19). Thus, we also examined whether sequential treatments incorporating therapeutic doses of retinoids and low concentrations of 4-HPR synergistically cause growth inhibitory effects in lung cancer cells, and whether these occur via RAR or RXR pathways.
The NSCLC H460, H1299, H1703, and A549 cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, U.S.A.). H1299, H1703, and H460 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), whereas A549 cells were cultured in F12 supplemented with 10% FBS. All cell lines were grown at 37℃ in a humidified 5% CO2 atmosphere. Cells were seeded at 5×105 cells/10-cm plate in 10% FBS-containing medium, but at 70% confluence were incubated for 24 hr in 0.5% FBS-containing medium, in order to minimize the effects of endogenous RA in culture medium. They were then treated with retinoids for 48 hr, washed twice in phosphate-buffered saline (PBS; pH 7.4), and collected by trypsinization.
ATRA, 13-cis RA, 9-cis RA, and 4-HPR were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.), dissolved in 100% DMSO at 100 mM or 10 mM (4-HPR), and stored in the dark at -80℃. We treated cell lines with these agents to evaluate interactions between 4-HPR and the other retinoids in the following ways: protocol 1 (0.1 µM 4-HPR alone), protocol 2 (1 µM ATRA alone), protocol 3 (0.1 µM 4-HPR for 1 hr followed by 1 µM of ATRA), protocol 4 (1 µM ATRA for 1 hr followed by 0.1 µM of 4-HPR), and protocol 5 (simultaneous treatment with 0.1 µM 4-HPR plus 1 µM ATRA). 13-cis RA or 9-cis RA were examined in the same manner as ATRA.
Cellular growth rates were determined using MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assays. Cells were grown in 96-well plates in 0.5% FBS-containing medium at an initial density of 5×103 cells/well. After 24 hr, wells were treated with various concentrations of retinoid. After 24, 48 or 72 hr, 50 µL of MTT solution (Sigma, St. Louis, MO, U.S.A.) was added, and plates were incubated at 37℃ for 4 hr. Media and treated solutions were then aspirated, and the formazan formed was solubilized with 150 µL DMSO. Cell viabilities were measured as recommended by the manufacturer, and individual well absorbances were measured at 540 nm using a microplate reader (BioRad, Hercules, CA, U.S.A.).
Total cellular RNA was isolated from cells treated with or without various concentrations and combinations of retinoids, using TriReagent-RNA isolation reagent (Molecular Research Center, Cincinnati, OH, U.S.A.), according to the manufacturer's instructions. Two-microgram aliquots of total RNA were used to generate cDNAs using Moloney murine leukemia virus reverse transcriptase (MMLV; Gibco/BRL, Gaithersburg, MD, U.S.A.) and Oligo-d (T)15 primer (Roche, Indianapolis, IN, U.S.A.) in a final volume of 20 µL. Reaction mixtures consisted of 5x first strand buffer (Gibco/BRL), 0.1 M DTT, 2.5 mM each dNTP, 2.5 units/µL MMLV, and 2.5 µM Oligo-d(T)15 primer. The cDNA products (1 µL) obtained were then subjected to PCR to amplify RARβ2 and β-actin. The sense and antisense primers used for RARβ2 were 5'-CAT GTT TGA CTG TAT GGA TG-3' and 5'-AGC CCT TAC ATC CCT CAC AG-3', respectively, which produced a 329-bp PCR product, and those for β-actin (the internal control) were 5'-ACC CAG ATC ATG TTT GAG ACC-3' and 5'-GGA GTT GAA GGT AGT TTC GTG-3', respectively, and produced a 486-bp PCR product. The PCR reaction mixture (50 µL) consisted of 1× reaction buffer, 2 mM MgCl2, 200 µM dNTP, 0.4 units Han-Taq polymerase (Genenmed, Seoul, Korea) and 0.2 µM of each primer. An initial denaturation at 94℃ for 5 min was followed by 35 amplification cycles of 94℃ for 30 sec, 50℃ for 30 sec, and 72℃ for 1 min and a final extension step at 72℃ for 10 min. The PCR products obtained from each sample were then electrophoresed on 2% agarose gel, stained with ethidium bromide, and photographed.
Median effect analysis, using the Hill equation, was used to determine synergistic, additive, and antagonistic effects, when up to three agents were combined (20). H1703 cells were incubated for 1 hr with different concentrations of ATRA or 13-cis RA (at 0.5, 1, 2, or 4 µM), and then incubated for 48 or 72 hr with 4-HPR (0.05, 0.1, 0.2 and 0.4 µM). The effects of sequential treatment with ATRA or 13-cis RA and 4-HPR were determined using a dose-effect analysis software (Biosoft, Cambridge, England). CI values indicated synergism (CI<1), an additive effect (CI=1), or antagonism (CI>1).
Total sample cellular RNAs (10 µg) were electrophoresed on 1% agarose gel, transferred overnight to GeneScreen Plus® nylon membranes (NEN®Life Science Products, Inc., Boston, MA, U.S.A.) by the capillary transfer method, and then immobilized on membranes by UV crosslinking. RNA blots were prehybridized and then hybridized to [α-32P]-dCTP-labeled RAR cDNA probes, which were kindly donated by Dr. Jonathan M. Kurie and Dr. Ronald Evans (the University of Texas, M.D. Anderson Cancer Center, Houston, Texas and the Salk Institute, San Diego, CA) (19-21). After incubation overnight, membranes were washed under high-stringency conditions (0.1X SSC at 65℃) and exposed overnight (below -70℃) to radiography film for autoradiography. Membranes were also stripped and reprobed with GAPDH cDNA, which was used as an RNA loading control. Quantitative analyses were performed with a Multi-Image light cabinet, and quantified using a Gel-doc Image analyzer (Bio-Rad, Hercules, CA, U.S.A.).
To compare the efficiencies of the retinoids, H460, H1299, H1703, or A549 cells were treated with increasing concentrations (0.1-10 µM) of retinoids for 24, 48, or 72 hr, and then MTT assayed. Growth inhibition was defined when treated cells had viabilities <70% of those of untreated cells. Weak or no growth inhibition was observed after treating either A549 or H1703 with low or high concentrations (0.1 or 1 µM, respectively) of 4-HPR (Table 1). In contrast, H1299 cell growth was inhibited by 4-HPR at both of these concentrations, whereas H460 cell growth was inhibited by 4-HPR at 1 µM only. At the clinically achievable concentration of 1 µM, no growth inhibition was observed by treatment of 9-cis RA, 13-cis RA, or ATRA in any of the four NSCLC cell-lines. Although the treatment with 9-cis, 13-cis RA, or ATRA at 10 µM inhibited cell growths by 30-84%, this concentration is difficult to achieve in human serum due to intolerable toxicity or rapid drug metabolism.
In order to determine whether retinoid treatment restores RARβ expression, RARβ mRNA levels in NSCLC cells treated with retinoids were assessed by RT-PCR. Since the pharmacologically achievable concentration of retinoids is 1 µM, this concentration was used for each retinoid. Although no growth inhibitory effect was observed after adding ATRA, 13-cis RA, or 9-cis RA at 1 µM, it was found that RARβ expression was up regulated in H460 and in H1299 cells treated with ATRA, 13-cis RA, or 9-cis RA at 1 µM (Fig. 1). Moreover, A549 cells revealed RARβ expression induction after treatment with 1 µM ATRA or 1 µM 9-cis RA, whereas 1 µM 13-cis RA failed to increase RARβ expression in these cells. H1299 cells and H460 cells, which showed growth inhibition after being treated with 1 µM 4-HPR, also showed RARβ expressional induction post-treatment (Table 1). Moreover, A549 cells, which were refractory to 1 µM 4-HPR treatment, showed brief RARβ expressional induction at 24 hr post-treatment. However, in 4-HPR refractory H1703 cells, which showed no basal RARβ expression, RARβ was not induced by 1 µM 4-HPR or by the other retinoids at this concentration. These results indicate that 1 µM 4-HPR may induce growth inhibition and induce RARβ expression in RARβ-positive NSCLC cells.
To investigate the growth inhibitory effects of combinational (sequential and simultaneous) treatments with 4-HPR and other retinoids, each of the NSCLC cell lines was treated using the sequential and simultaneous protocols described in 'Materials and Methods'. All NSCLC cell lines showed growth inhibition when exposed to 1 µM ATRA followed by 0.1 µM 4-HPR (Fig. 2A). In particular, sequential treatment with ATRA or 13-cis RA and 4-HPR (ATRA or 13-cis RA 1 µM for 1 hr followed by 4-HPR 0.1 µM for 48 hr) markedly inhibited the growth of H1703 cells, which exhibited no basal RARβ expression and were refractory to 4-HPR treatment. These results imply that the growth inhibitory effects of 4-HPR can be facilitated by pretreating first with other retinoids that regulate the expressions of RARs or RXRs.
In order to confirm the synergistic effects of sequential ATRA or 13-cis RA and 4-HPR, combination index (CI) plots were drawn. Table 2 and 3 depict fractions affected (Fa) and CI values. Plots of CI versus Fa are shown for sequential 13-cis RA or ATRA in combination with 4-HPR (ATRA or 13-cis RA 1 µM for 1 hr followed by 4-HPR 0.1 µM for 48 hr or 72 hr) in H1703 cells (Fig. 3). ATRA or 13-cis RA for 1 hr followed by 4-HPR, especially for 72 hr of 4-HPR treatment showed consistent synergism with various combinations of ATRA or 13-cis RA with CI values of <1, indicating synergism.
In order to determine whether combinational treatments of 4-HPR and other retinoids enhance RAR or RXR expressions, northern blot analyses were performed (Fig. 4); results were compared with those obtained for single treatments. ATRA 1 µM treatment induced RARα expression in all NCSLC cell lines and induced RARβ in three cell lines (except H1703). In the other NSCLC cell lines, which showed basal RARβ expression, RARβ expressional levels were up regulated by 1 µM ATRA alone or by sequentially treating with 1 µM ATRA and 0.1 µM 4-HPR (Fig. 4B). In contrast, RARγ expression was induced by 1 µM ATRA in all NSCLC cell lines, but these inductions of RARγ were lost by simultaneously or sequentially treating ATRA and 4-HPR in all four NSCLC cell lines (Fig. 4C).
Basal RARβ-positive NSCLC cell lines revealed RARγ expressional induction after 0.1 µM 4-HPR treatment, whereas H1703 cells, which did not exhibit RARβ basal expression, showed reduced RARγ expression after treatment with 0.1 µM 4-HPR. The synergistic growth inhibition shown by H1703 cells sequentially treated with ATRA 1 µM for 1 hr followed by 4-HPR 0.1 µM for 48 hr was accompanied by reduced RARγ expression. Moreover, this sequential treatment with 4-HPR prevented the upregulation of RARγ by ATRA. These findings indicate that sequential treatment with other retinoids and 4-HPR may cause growth inhibition due to the modulation of RAR expressions by the initially treated retinoids.
The retinoids are a class of natural and synthetic vitamin A analogues, and some retinoids are known to play major roles in cell growth regulation and in the differentiation of normal, benign, and malignant cell types. Retinoids are among the most intensively studied cancer chemoprevention agents. However, the broad biological activities of the retinoids lead to a number of undesirable side effects, which limits their long-term clinical usefulnesses as chemopreventatives (21).
13-cis RA was found to be effective at reversing upper aerodigestive tract premalignancies (22) and at preventing second primary tumor development in advanced head and neck cancer (23), but its administration was also associated with significant toxicity. In terms of lung cancer treatment, the United States Intergroup Study found no benefit for 13-cis RA intervention in individuals with a prior history of lung cancer (8). In the present study, no growth inhibitory effects were observed on therapeutic (1 µM) dosages of ATRA or 13-cis RA, although RARβ expression was found to be up-regulated in NSCLC cell lines after ATRA or 13-cis RA treatment.
9-cis RA, a natural ATRA isomer, is a pan-agonist that is able to bind to and activate both RXRs and RARs. 9-cis RA can activate RXR/RAR heterodimers, RXR/RXR homodimers, and other nuclear receptor complexes in which RXR is a ligand-binding partner, such as, vitamin D receptor and peroxisome proliferator-activated receptors (24). Thus, by virtue of its unique receptor-binding properties, 9-cis RA exerts biological effects that differ from those of ATRA or 13-cis RA. In a recent study, 9-cis RA was found to up regulate RARβ expression in bronchial squamous metaplasia (11), which suggests that 9-cis RA is a potential chemopreventive agent in NSCLC. However, in the present study, 9-cis RA inhibited NSCLC cell growth only at higher than clinically acceptable concentrations.
4-HPR, despite its inability to bind directly to nuclear receptors, shows some preventive activity in experimental animals, and was also found to be active in lung cancer cell lines, where it inhibited growth and induced apoptosis. However, 4-HPR does not induce cell differentiation as in RAs, but rather induces apoptosis (12, 25, 26), which is not effectively induced by RAs. ROS production, the RAR/RXR pathway, and a variety of mechanisms yet to be elucidated can mediate 4-HPR-induced apoptosis. Moreover, several previous reports have indicated that 4-HPR is far more potent than ATRA in terms of inducing NSCLC cell apoptosis, thus indicating the need for further preventative and therapeutic trials (18). Although in vitro studies have demonstrated that 4-HPR (at concentrations ranging from 1 to 10 µM) suppresses the growth of NSCLC cells, and that these effects are associated with apoptotic induction, 4-HPR was not effective at reversing squamous metaplasia, dysplasia, or genetic and phenotypic abnormalities in the bronchial epithelium of smokers (27). This inactivity of 4-HPR with respect to pathologic changes in bronchial mucosa raised the possibility that serum 4-HPR levels in this previous study were too low to achieve a biological effect. The apoptotic effects of 4-HPR in tissue cultures typically require 4-HPR concentrations of >1 µM (18). A phase I trial of 4-HPR in children with neuroblastoma demonstrated that high-dose 4-HPR was well tolerated up to 4,000 mg/m2/day (19). The highest dose examined in this trial produced an average drug plasma level of 12.9 µM, and an association was found between plasma peak levels and toxicity-associated side effects. After repeated treatments, plasma peak concentrations of <3 µM appeared to be safe. Moreover, the frequency of grade 2-3 toxicity was found to be significantly higher in patients with peak levels exceeding 3 µM (19). In the present study, the growth inhibitory effect of 4-HPR in NSCLC cell lines was interesting, because, although no growth inhibitory effects were observed in A549 or H1703 cells with 0.1 µM treatment, 4-HPR inhibited the growths of all four NSCLC cell lines tested at the clinically relevant concentration of 1 µM, and in fact, H1299 cells were even inhibited at 0.1 µM.
Recently, ROS generation has been implicated in 4-HPR-induced apoptosis in some malignant cells (28). Low doses of ROS, particularly of hydrogen peroxide, are known to be mitogenic and to promote cell proliferation, whereas high levels of oxidative stress ultimately cause cell death via apoptotic or necrotic mechanisms (29). Thus, if 4-HPR induces apoptosis primarily by inducing ROS production, 4-HPR could have antagonistic effects when co-administered with ATRA, 13-cis RA, or 9-cis RA, as these species are antioxidants (14). Conversely, if 4-HPR mainly acts on RAR, combinations of 4-HPR and other retinoids may act synergistically.
4-HPR binds to retinol-binding protein in the liver, and therefore competes with retinol and reduces serum retinol levels (30). Thus, loss of serum retinol may counterbalance the chemopreventive effects of 4-HPR, and the effect of 4-HPR might be enhanced by restoring normal retinol levels by administering 4-HPR in combination with other retinoids (27).
The growth inhibitory effects of retinoid treatments in all NSCLC cells examined were greater when the cells were exposed to 1 µM ATRA followed by 0.1 µM 4-HPR. H1703 cells, which do not show RARβ basal expression, were not sensitive to 4-HPR treatment alone. However, sequential treatment with ATRA or 13-cis RA followed by 4-HPR synergistically inhibited H1703 cell growth. Moreover, these growth inhibitions were accompanied by reduced RARγ expression on H1703 cells, when the cells were treated with 4-HPR alone or with sequential ATRA or 13-cis RA and 4-HPR. RARγ expression also was lower in cells treated with sequential ATRA/4-HPR than in cells treated with ATRA alone in all NSCLC cell lines. Since these cells showed RARγ up regulation after a single ATRA treatment, 4-HPR appears to act in concert with the up regulated RARγ expression induced by ATRA but to override the effect of ATRA when they are administered sequentially. The ATRA/4-HPR combination has been examined in other cancer cells. Lim et al. investigated whether 4-HPR (due to its suppression of HER2/neu and/or EGFR expression) sensitized breast cancer cells to ATRA, and found that at a concentration of 1.3 µM, 4-HPR increased ATRA sensitivity in a synergistic manner in BT-474, MDA-MB-453, and MCF-7/HER2 breast cancer cells (31). Another study demonstrated the efficacy of 4-HPR in mice bearing the human ovarian carcinoma IGROV-1, and demonstrated that 4-HPR significantly enhanced the antitumor activity of cisplatin. In IGROV-1 tumor-bearing mice, a simultaneous RA/4-HPR combination was found to significantly improve the efficacy of 4-HPR, and resulted in an antitumor activity similar to that of cisplatin alone. These findings demonstrate that the simultaneous RA/4-HPR combination may increase the antitumor activity of 4-HPR (32).
We conclude that sequential ATRA or 13-cis RA and 4-HPR treatments offer a valid therapeutic approach to the treatment or chemoprevention of NSCLC via RAR expressional modulation. Moreover, this approach could overcome 4-HPR refractoriness in NSCLC cells. Further work is warranted to determine the identities of the molecular signals underlying the synergistic and additive effects of RAs.
Figures and Tables
 | Fig. 1RARβ mRNA expression by RT-PCR. Cells were pretreated with 4-HPR (A), 9-cis RA (B), 13-cis RA (C), or ATRA (D) at 1 µM for 48 hr. Total RNA was extracted after treating cells with each of the indicated agents for 48 hr, and the cDNA generated by MMLV reverse transcriptase was subjected to PCR using specific primers for RARβ messenger RNA (329 bp). The procedures used for total RNA isolation are described in 'Materials and Methods'. β-actin was used as an internal control. Amplified DNA was separated on 2% agarose gel and stained with ethidium bromide. |
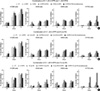 | Fig. 2Effects of combinational treatments with 4-HPR and other RAs (A), 13-cis RA (B), or 9-cis RA (C). NSCLC cell lines were treated with RAs as described in 'Materials and Methods'. Cell survivals were determined using MTT assays. Experiments were repeated 3 times to ensure reproducibility. Error bars are the means of 3 independently performed experiments and are shown with their respective standard deviations. |
 | Fig. 3Median effect plots of the cytotoxic effects (Fa) of sequential 13-cis RA or ATRA and 4-HPR treatment in H1703 cells. (A) H1703 cells were treated with 13-cis RA for 1 hr and then incubated for 48 hr (◆) or 72 hr (■) with 4-HPR. (B) H1703 cells were treated with ATRA for 1 hr and then incubated for 48 hr (◆) or 72 hr (■) with 4-HPR. Combination index (CI) values of <1 represent synergy between ATRA and 13-cis RA or between ATRA and 4-HPR, CI values >1 indicate antagonism and values of 1 indicate an additive effect. |
 | Fig. 4Northern analysis of the expressions of RARs after treating NSCLC cell lines with 4-HPR and other retinoids (ATRA, 13-cisRA, or 9-cisRA) singly or in combination. Cells were treated as described above. For Northern analysis, 10 µg of total RNA was subjected to electrophoresis in agarose gel and blotted onto a nylon membrane. H460 was a RAR positive control. GAPDH was used as an internal loading control. Blotting procedures are described in 'Materials and Methods'. Densities (%) of the RAR expressions are expressed as density ratios versus control (CT) levels. |
References
1. Lotan R. Retinoids as modulators of tumor cells invasion and metastasis. Semin Cancer Biol. 1991. 2:197–208.
2. Hofmann SL. Retinoids--"differentiation agents" for cancer treatment and prevention. Am J Med Sci. 1992. 304:202–213.

3. McBurney MW, Costa S, Pratt MA. Retinoids and cancer: a basis for differentiation therapy. Cancer Invest. 1993. 11:590–598.

4. Fanjul AN, Delia D, Pierotti MA, Rideout D, Yu JQ, Pfahl M. 4-Hydroxyphenyl retinamide is a highly selective activator of retinoid receptors. J Biol Chem. 1996. 271:22441–22446.

5. Lokshin A, Zhang H, Mayotte J, Lokshin M, Levitt ML. Early effects of retinoic acid on proliferation, differentiation and apoptosis in non-small cell lung cancer cell lines. Anticancer Res. 1999. 19:5251–5254.
6. Treat J, Friedland D, Luginbuhl W, Meehan L, Gorman G, Miller W Jr, Bavaria J, Kaiser L. Phase II trial of all-trans retinoic acid in metastatic non-small cell lung cancer. Cancer Invest. 1996. 14:415–420.

7. Lippman SM, Hong WK. 13-cis-retinoic acid and cancer chemoprevention. J Natl Cancer Inst Monogr. 1992. 111–115.
8. Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, Khuri FR, Marks R, Winn RJ, Fry W, Graziano SL, Gandara DR, Okawara G, Woodhouse CL, Williams B, Perez C, Kim HW, Lotan R, Roth JA, Hong WK. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001. 93:605–618.

9. Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992. 68:397–406.

10. Mernitz H, Smith DE, Zhu AX, Wang XD. 9-cis-Retinoic acid inhibition of lung carcinogenesis in the A/J mouse model is accompanied by increased expression of RAR-beta but no change in cyclooxygenase-2. Cancer Lett. 2006. 244:101–108.
11. Kurie JM, Lotan R, Lee JJ, Lee JS, Morice RC, Liu DD, Xu XC, Khuri FR, Ro JY, Hittelman WN, Walsh GL, Roth JA, Minna JD, Hong WK. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-beta expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003. 95:206–214.
12. Ohlmann CH, Jung C, Jaques G. Is growth inhibition and induction of apoptosis in lung cancer cell lines by fenretinide [N-(4-hydroxyphenyl)retinamide] sufficient for cancer therapy? Int J Cancer. 2002. 100:520–526.

13. Sabichi AL, Xu H, Fischer S, Zou C, Yang X, Steele VE, Kelloff GJ, Lotan R, Clifford JL. Retinoid receptor-dependent and independent biological activities of novel fenretinide analogues and metabolites. Clin Cancer Res. 2003. 9:4606–4613.
14. Um SJ, Lee SY, Kim EJ, Han HS, Koh YM, Hong KJ, Sin HS, Park JS. Antiproliferative mechanism of retinoid derivatives in ovarian cancer cells. Cancer Lett. 2001. 174:127–134.

15. Supino R, Crosti M, Clerici M, Warlters A, Cleris L, Zunino F, Formelli F. Induction of apoptosis by fenretinide (4HPR) in human ovarian carcinoma cells and its association with retinoic acid receptor expression. Int J Cancer. 1996. 65:491–497.

16. Kim HJ, Chakravarti N, Oridate N, Choe C, Claret FX, Lotan R. N-(4-hydroxyphenyl)retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006. 25:2785–2794.

17. Sun SY, Li W, Yue P, Lippman SM, Hong WK, Lotan R. Mediation of N-(4-hydoxyphenyl)retinamide-induced apoptosis in human cancer cells by different mechanisms. Cancer Res. 1999. 59:2493–2498.
18. Zou CP, Kurie JM, Lotan D, Zou CC, Hong WK, Lotan R. Higher potency of N-(4-hydroxyphenyl)retinamide than all-trans-retinoic acid in induction of apoptosis in non-small cell lung cancer cell lines. Clin Cancer Res. 1998. 4:1345–1355.
19. Garaventa A, Luksch R, Lo Piccolo MS, Cavadini E, Montaldo PG, Pizzitola MR, Boni L, Ponzoni M, Decensi A, De Bernardi B, Bellani FF, Formelli F. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003. 9:2032–2039.
20. Budman DR, Calabro A. Studies of synergistic and antagonistic combinations of conventional cytotoxic agents with the multiple eicosanoid pathway modulator LY 293111. Anticancer Drugs. 2004. 15:877–881.

21. De Luca LM. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991. 5:2924–2933.

22. Khuri FR, Cohen V. Molecularly targeted approaches to the chemoprevention of lung cancer. Clin Cancer Res. 2004. 10:4249s–4253s.

23. Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, Dimerg IW, Brown BW, Goepfert H. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990. 323:795–801.

25. Delia D, Aiello A, Formelli F, Fontanella E, Costa A, Miyashita T, Reed JC, Pierotti MA. Regulation of apoptosis induced by the retinoid N-(4-hydroxyphenyl) retinamide and effect of deregulated bcl-2. Blood. 1995. 85:359–367.

26. Pellegrini R, Mariotti A, Tagliabue E, Bressan R, Bunone G, Coradini D, Della Valle G, Formelli F, Cleris L, Radice P, Pierotti MA, Colnaghi MI, Ménard S. Modulation of markers associated with tumor aggressiveness in human breast cancer cell lines by N-(4-hydroxyphenyl) retinamide. Cell Growth Differ. 1995. 6:863–869.
27. Kurie JM, Lee JS, Khuri FR, Mao L, Morice RC, Lee JJ, Walsh GL, Broxson A, Lippman SM, Ro JY, Kemp BL, Liu D, Fritsche HA, Xu X, Lotan R, Hong WK. N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res. 2000. 6:2973–2979.
28. Sun SY, Yue P, Lotan R. Induction of apoptosis by N-(4-hydroxyphenyl) retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors, and apoptosis-related genes in human prostate carcinoma cells. Mol Pharmacol. 1999. 55:403–410.
29. Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002. 192:1–15.

30. Holven KB, Natarajan V, Gundersen TE, Moskaug JO, Norum KR, Blomhoff R. Secretion of N-(4-hydroxyphenyl) retinamide-retinolbinding protein from liver parenchymal cells: evidence for reduced affinity of the complex for transthyretin. Int J Cancer. 1997. 71:654–659.

31. Lim SJ, Gutierrez-Puente Y, Tari AM. N-(4-hydroxyphenyl)-retinamide selectively increases All-TRANS retinoic acid inhibitory effects in HER2/NEU-overexpressing breast cancer cells. Tumour Biol. 2002. 23:279–286.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download