Abstract
The RUNX3 gene is regarded as a tumor suppressor gene in many human solid tumors, and its inactivation is believed to be related with solid tumor carcinogenesis. As little information is available about the role of the RUNX3 gene in breast cancer, we investigated the relationship between the RUNX3 gene and breast cancer. We performed reverse transcriptase-polymerases chain reaction (RT-PCR), methylation specific PCR, and bicolor fluorescent in situ hybridization analysis in an effort to reveal related mechanisms. Forty breast tissue samples and 13 cell lines were used in this study. Eighty-five percent of breast cancer tissues showed downregulated RUNX3 gene expression, whereas it was downregulated in only 25% of normal breast tissues by RT-PCR assay. Sixty-seven percent of breast cancer cell lines showed downregulated RUNX3 expression, but the RUNX3 gene was not expressed in two normal breast cell lines. Hypermethylation was observed in 53% of breast cancer tissues and 57% of breast cancer cell lines. Hemizygous deletion was observed in 43% of breast cancer cell lines. Hypermethylation and/or hemizygous deletion was observed in 5 of 7 breast cancer cell lines, and the four of these five examined showed no RUNX3 gene expression. We suggest that various mechanisms, including methylation and hemizygous deletion, could contribute to RUNX3 gene inactivation.
Recently, it has been reported that RUNX3, a RUNX family gene, is related to the carcinogeneses of various solid human tumors. The RUNX (Runt-related transcription factor) gene family is also referred to as the acute myeloid leukemia (AML), core-binding factor-α (CBFα), or polyoma enhancer-binding protein-2α (PEBP2α) family (1) and encodes the α-subunit of PEBP2/CBF (2). Moreover, the heterodimeric RUNX-CBFβ complex is believed to be associated with cellular differentiation and proliferation (3, 4). Three RUNX family genes, i.e., RUNX1 (AML1/CBFA2/PEBP2aB), RUNX2 (AML3/CBFA1/PEBP2aA), and RUNX3 (AML2/CBFA3/PEBP2aC) have been detected in mammals (1). RUNX1 is believed essential in hematopoiesis (5) and is located in chromosome 21q22.3 (6). Moreover, it is targeted for translocation (7) or mutation (8) in acute and chronic leukemia or myelodysplastic syndrome. RUNX2 on chromosome 6q21 (9) is regarded as indispensable for the development of the musculoskeletal system (10) and has been related with cleidocranial dysplasia (11). On the other hand, RUNX3 gene is located on chromosome 1p36, 30.31 kb-sized (1), composed of 5 exons (12), and is believed to be associated with solid tumor carcinogenesis.
Although many have reported an association between RUNX3 inactivation and solid tumor carcinogenesis, e.g., gastric cancer (13-17), colorectal cancer (18, 19), lung cancer (20-22), esophageal cancer (23), hepatocellular carcinoma (24-26), pancreatic cancer (27), and yolk sac tumor (28), others found no such correlations (29).
Several mechanisms including promoter region hypermethylation, loss of heterozygosity, hemizygous deletion, and mutation are believed to be correlated with the downregulation of the RUNX3 gene, and have been causally linked with the carcinogeneses in a wide range of human solid tumors.
Little information is available on the relationship between the RUNX3 gene and breast cancer, other than the hypermethylation that was observed in 25% of 25 breast cancer tissues by Kim et al. (15). In order to reveal the relationship between the RUNX3 gene and breast cancer and its mode of action, we performed reverse transcriptase-polymerase chain reaction (RT-PCR), methylation-specific PCR (MSP) and bicolor fluorescent in situ hybridization (FISH) on 40 breast tissues and 13 cell lines.
Forty breast tissue samples (20 normal breast tissues and 20 breast cancer tissues) were collected for this study. These tissue samples were obtained during resective surgery, immediately frozen in liquid nitrogen, and stored at -80℃ until required for DNA and RNA extraction. Breast cancer tissue samples were macrodissected through the centers of carcinomatous regions and then normal breast tissue samples, well separate from cancer bearing regions, were macrodissected. The pathologic classification of all 20 breast cancer tissues was of infiltrating ductal carcinoma. This study was performed with given patient consent at Seoul National University Hospital.
Thirteen cell lines were used in this study. Eight cell lines (Hs 578T, MCF-7, MDA-MB-231, SCC-1395, SK-BR-3, ZR-75-1, SNU1, and SNU5) were obtained from the Korean Cell Line Bank (Seoul), and the other five (MCF10A, MCF-7 TH, MDA-MB-468, MDA-MB-436, and T-47D) from the American Type Culture Collection (ATCC). Two cell lines, SCC-1395 and MCF 10A, were used as non-cancer controls. These two cell lines originated from normal breast tissue and breast tissue showing fibrocystic change, respectively. Another two cell lines, SNU1 and SNU5, originated from gastric carcinoma. Because the SNU1 cell line is known to have only methylated DNA products by MSP, it was used as a positive control for MSP. Similarly, because the SNU5 cell line has only unmethylated DNA products, it was used as a negative control. The remaining 9 cell lines originated from breast cancer (ZR-75-1, MCF 7, SK-BR-3, Hs 578T, MDA-MB-231, T-47D, MDA-MB-436, MDA-MB-468, and MCF-7 TH).
Seven cell lines, namely, SCC-1395, ZR-75-1, MCF7, T-47D, MCF-7 TH, SNU1, and SNU5, were cultured in Roswell Park Memorial Institute (RPMI) 1640 enriched with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). MDA-MB-231, MDA-MB-436, and Hs 578T were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 1% penicillin/streptomycin and 10% FBS. SK-BR-3 and MDA-MB-468 were cultured in DMEM/F-12 (Nutrient Mixture F-12) containing 1% penicillin/streptomycin and 10% FBS, and MCF 10A was cultured in DMEM/F-12 containing 1% penicillin/streptomycin, 5% horse serum (all of the above were purchased from GibcoBRL, NY, U.S.A.), 10 µg/mL insulin, 10 ng/mL epithelial growth factor, 0.5 ng/mL hydrocortisone, and 0.1 µg/mL cholera toxin (Sigma-Aldrich, St. Louis, MO). Cell culture plates were maintained in humidified incubators at 37℃ in a 5% CO2 atmosphere.
Total RNA was extracted from breast cancer tissues and cell lines using TRIzol® reagent (Invitrogen, CA, U.S.A.), and complementary DNA was synthesized from total RNA with deoxyribonucleotide triphosphate (dNTP) mix, RNase I (Ribonuclease Inhibitor), dithiothreitol (DTT), diethylpyrocarbonate (DEPC), RNA, 5 X first-stand buffer, Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Invitrogen) and random primer. Complementary DNA was amplified using a RUNX3 gene specific primer with complementary DNA template, dNTP, Taq polymerase, distilled water (DW), and 10X buffer. Initial denaturation was performed at 94℃ for 5 min, and this was followed by 35 amplification cycles (30 sec at 95℃, 55℃, and 72℃). Final elongation was performed at 72℃ for 10 min, and β-actin was used as a control.
The primers used to amplify RUNX3 and β-actin were as follows: sense for RUNX3, 5'-GAG TTT CAC CCT GAC CAT CAC TGT G-3'; antisense for RUNX3, 5'-GCC CAT CAC TGG TCT TGA AGG TTG T-3' (869 base pairs); sense for β-actin, 5'-CAC TGT GTT GGC GTA CAG GT-3'; antisense for β-actin, 5'-TCA TCA CCA TTG GCA ATG AG-3' (155 base pairs). Electrophoresis was followed by visualization under ultraviolet light after loading the amplified PCR products on 2% agarose gel containing ethidium bromide.
We classified the level of RUNX3 expression into 3 types such as weak, moderate, and strong expression in comparison with the expression level of β-actin. Strong expression means that the level of RUNX3 expression is almost same with that of β-actin. When the expression of RUNX3 is visible but very faint, we classified it as weak expression. If the expression of RUNX3 is intermediate between strong and weak, then we regard it as moderate expression.
One Day MSP kits (In2Gen Co., Seoul) were used to determine the promoter methylation status of the RUNX3 gene in genomic DNA extracted from breast cancer tissues and cell lines. Initial denaturation was performed at 95℃ for 15 min followed by 40 amplification cycles of; 95℃ (20 sec), 60℃ (40 sec), and 72℃ (60 sec). Final elongation was performed at 72℃ for 3 min.
The following primers were used as previously described by Li et al. (13): sense for methylation, 5'-TTA CGA GGG GCG GTC GTA CGC GGG-3'; antisense for methylation, 5'-AAA ACG ACC GAC GCG AAC GCC TCC-3' (220 base pairs); sense for non-methylation, 5'-TTA TGA GGG GTG GTT GTA TGT GGG-3'; antisense for non-methylation, 5'-AAA ACA ACC AAC ACA AAC ACC TCC-3' (212 base pairs). After electrophoresis on 2% agarose gel containing ethidium bromide, the amplified PCR products were visualized under ultraviolet light.
Cells (1×106/mL) fixed with Carnoy's solution (methanol: acetic acid, 3:1) were mixed with 0.1% Nonidet P-40 (NP-40)/2× standard saline citrate (SSC) solution for 30 min to increase cell membrane permeabilities and dehydrated for 3 min in a 70%, 85%, 100% ethanol series. All of the following procedures were performed in the dark. A probe mixture prepared from RUNX3 gene-specific probe tagged with Cy3, and a 1q21.1 specific probe tagged with FITC (control) was mixed with human Cot-1 DNA, salmon sperm DNA, and hybridization buffer. Denaturation and hybridization (DAKO, Hamburg, Germany) were performed after dropping a probe mixture on a slide containing cells followed by DAPI solution for background staining. The RUNX3 gene-specific probe was tagged with Cy3 release orange and the 1q21.1 specific probe was tagged with FITC release green.
After performing the above, 100 cells from each cell lines were randomly observed. If the green staining exceeded the orange staining in a single cell, then the O/G (O and G mean orange and green staining, respectively) ratio was defined to be less than 1. If O/G ratios were less than 1 in more than 20% of observed cells, then hemizygous deletion of the RUNX3 gene was presumed in the affected cell line.
RUNX3 gene expression was not observed in five (numbers 3, 8, 14, 17, and 19) of the 20 normal breast tissue samples, but only three samples (numbers 4, 5, and 9) of 20 breast cancer tissues showed RUNX3 gene expression (Fig. 1, Table 1). RUNX3 gene expression was observed in six cell lines, namely, MDA-MB-436, SCC-1395, T-47D, ZR-75-1, MCF 10A, and SNU-5 (Table 2), and whereas SNU-5 showed strong expression, three cell lines, i.e., MDA-MB-436, T-47D, and ZR-75-1, showed weak expression. Moderate expression was observed in SCC-1395 and MCF 10A (Fig. 2). The RT-PCR product sizes for the RUNX3 and β-actin genes were 869 and 155 base pairs, respectively.
MSP results were available in 19 of the 20 breast cancer tissues. Sample number 19 was excluded because of the insufficient amount of specimen for repeated experiments. Methylated DNA products were observed in 10 breast cancer tissues (samples 2-4, 6, 8, 10, 11, 13, 14, and 15) and unmethylated DNA products in 18 breast cancer tissues (excepting only sample 13). Nine breast cancer tissues (samples 2-4, 6, 8, 10, 11, 14, and 15) showed both methylated and unmethylated DNA products (Fig. 3). Ten out of thirteen cell lines showed the results of MSP, and results were inadequate for three cell lines, i.e., SCC-1395, Hs 578T, and T-47D. Methylated DNA products were observed in 6 cell lines, i.e., MCF7, SNU-1, MDA-MB-231, SK-BR-3, MCF-7 TH, and MCF 10A, and unmethylated DNA products in 5 cell lines, i.e., MDA-MB-436, MDA-MB-468, SK-BR-3, ZR-75-1, and SNU-5. Methylated and unmethylated DNA products were only observed in SK-BR-3 (Fig. 4). The product sizes of the methylation and unmethylation MSP primers were 220 and 212 base pairs, respectively.
Bicolor FISH results were obtained in 9 cell lines, except SCC-1395, Hs 578T, T-47D, and MCF-7 TH (Table 3). These results were classified into 5 different patterns, i.e., normal, O/G<1, O/G>1, hypoploid, and hyperploid. If one, two, or more than 2 pairs of orange and green colors were observed in a single cell, then the cell was classified as hypoploid, normal, and hyperploid, respectively. If the amount of green was greater or smaller than the amount of orange, then the cell of interest was classified as O/G<1 or O/G>1, respectively. Because more than 20% of cells showed O/G<1 for the MB 436, MCF7, SK-BR-3, SNU-1, and SNU-5 cell lines, hemizygous deletion of the RUNX3 gene was deemed to be present in these cell lines. Less than 20% of cells showed O/G<1 in ZR-75-1, MDA MB 231, and MDA MB 468, and no cell had an O/G<1 in MCF 10A. All MCF 10A cells and 95% of ZR-75-1 cells showed a normal pattern, whereas all MCF7 cells had an O/G of <1 (Fig. 5). Only 2 cell lines, i.e., MDA MB 468 and SNU-1, had an O/G of >1, and only MDA MB 436 showed a hypoploid pattern. Seven cell lines, except MCF 10A and MCF7, showed various degrees of hyperploidy.
Recently it was reported that RUNX3 gene expression is downregulated in various solid tumors in vivo. While Li et al found that RUNX3 gene expression was downregulated in 60% of gastric cancer tissues and in 47% of gastric cancer cell lines (13), Sakakura et al. found that RUNX3 gene expression was downregulated in 78% of gastric cancer tissues and in 69% of gastric cancer cell lines (14). Regarding colorectal cancer cell lines, Ku et al. reported that 50% showed decreased RUNX3 gene expression (18), and Goel et al. found that RUNX3 gene expression was downregulated in 65% of colorectal cancer cell lines (19). Other studies have reported various degrees of RUNX3 downregulation in lung cancer, esophageal cancer, hepatocellular carcinoma, pancreatic cancer, and others. However, little data is available on the relationship between RUNX3 gene expression and breast cancer.
In the present study, 85% of breast cancer tissues (17 of 20 samples) showed RUNX3 gene downregulation, whereas it was downregulated in only 25% of normal breast tissues (5 of 20) by RT-PCR. Regarding the cell lines, 67% of breast cancer cell lines (6 of 9) showed RUNX3 downregulation, although in two normal breast cell lines (SCC-1395 and MCF 10A) RUNX3 gene expression was not detected. SNU-5 is known to possess only unmethylated DNA and showed strong RUNX3 expression, and SNU-1 is known to possess only methylated DNA and showed no RUNX3 expression. Moreover, while SCC-1395 and MCF 10A showed moderate expression, 3 of 9 breast cancer cell lines (MDA-MB-436, T-47D, and ZR-75-1) showed weak expression. These results imply that RUNX3 expression is downregulated in breast cancer and that this downregulation is related its carcinogenesis, as described for other cancers above.
Several genetic and epigenetic events may explain the mechanism underlying RUNX3 downregulation, such as, promoter region hypermethylation, loss of heterozygosity, microsatellite instability, hemizygous deletion, and mutation. Li et al found loss of heterozygosity in 30% of gastric cancer tissues and 20% of gastric cancer cell lines, and that hypermethylation is related to RUNX3 gene expression (13). They also have found one mutation in the RUNX3 gene among 119 gastric cancer tissue samples. Ku et al. have reported hypermethylation in 18.4% of colorectal cancer tissues and in 37.5% of colorectal cancer cell lines. Yanada et al. also found hypermethylation in 53% of lung cancer cell lines and loss of heterozygosity in 53% of lung cancer cell lines (22). Especially, promoter region hypermethylation was of very wide range from 2.5-73% in various tumor samples. Little information is available on breast cancer, but Kim et al. reported their observation of hypermethylation in 25% of 25 breast cancer tissues (15). In the present study, hypermethylation was observed in 53% (10 of 19) of breast cancer tissues and in 57% (4 of 7) breast cancer cell lines, and hemizygous deletion was observed in 43% (3 of 7) breast cancer cell lines. The results of our study on breast tissue and cell lines are summarized in Table 1, 2.
In breast cancer tissues, methylated DNA was found in 10 of 19 breast cancer tissues (53%), and RUNX3 gene expression was not observed in these samples except for one sample (sample 4) by RT-PCR (Table 1). Unmethylated DNA was observed in 18 breast cancer tissues (95%) except for sample 13, which showed only methylated DNA. Nine samples (47%) showed both methylated and unmethylated DNA. Although nine breast cancer tissues (47%) had only unmethylated DNA, the expression of RUNX3 gene was observed in only 2 of these samples. In addition, sample 4 showed RUNX3 expression despite having methylated DNA. These results suggest that a mechanism other than methylation of RUNX3 promoter influences RUNX3 gene expression. With regard to cell lines, MSP results were available for 10 cell lines and bicolor FISH results for 9 cell lines (Table 2). Methylated DNA was observed in 6 cell lines (MCF 10A, MCF7, SK-BR-3, MDA-MB-231, MCF-7 TH, and SNU-1), whereas four cell lines (ZR-75-1, MDA-MB-436, MDA-MB-468, and SNU-5) contained only unmethylated DNA. Only SK-BR-3 showed both methylated and unmethylated DNA. Hemizygous deletion was observed in 5 cell lines (MCF7, SK-BR-3, MDA-MB-436, SNU-1, and SNU-5), and hypermethylation and/or hemizygous deletion in five cell lines (MCF7, SK-BR-3, MDA-MB-231, MDA-MB-436, and MCF-7 TH) of 7 breast cancer cell lines with available results, and 4 of these 5 cell lines did not show RUNX3 expression. Despite the hemizygous deletion in MDA-MB-436 (which contained only unmethylated DNA), RUNX3 expression was observed. In the case of MDA-MB-468, which contained only unmethylated DNA without hemizygous deletion, RUNX3 expression was not apparent. These results imply that a mechanism other than hypermethylation or hemizygous deletion participates in RUNX3 gene expression.
In conclusion, RUNX3 gene expression was found to be downregulated in breast cancer. In addition, it is evident that several mechanisms of RUNX3 gene inactivation, including methylation and hemizygous deletion, contribute to the development of breast cancer.
Figures and Tables
Fig. 1
RT-PCR analysis of RUNX3 gene expression in normal and breast cancer tissues. RUNX3 downregulation was observed in 5 of 20 normal breast tissues (A), but in 17 of 20 breast cancer tissues (B).

Fig. 2
RT-PCR analysis of RUNX3 gene expression in 13 breast cell lines. RUNX3 gene expression was observed in six cell lines (MDA-MB-436, SCC-1395, T47D, ZR-75-1, MCF10A, and SNU-5). SNU-5 showed strong expression, whereas SNU-1 showed none. SCC-1395 and MCF10A showed moderate expression, and MDA-MB-436, T47D, and ZR-75-1 showed weak expression.

Fig. 3
Methylation analysis of the RUNX3 gene in breast cancer tissues. The results of MSP were available for 19 samples (except sample 19; data for sample 14 is not shown in this figure.) Methylated DNAs were found in 10 samples (samples 2, 3, 4, 6, 8, 10, 11, 13, 14, and 15), and all except one (sample number 13) contained unmethylated DNAs. Nine breast cancer tissues (samples 2, 3, 4, 6, 8, 10, 11, 14, and 15) contained both methylated and unmethylated DNA. U and M denote unmethylation and methylation, respectively. N denotes normal human genomic DNA, which was used as an unmethylated PCR control, and DW is an abbreviation for distilled water, which was used as a negative PCR control.
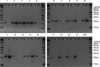
Fig. 4
Methylation analysis of the RUNX3 gene in cells. MSP results were available for 10 cell lines (except SCC-1395, Hs 578T, and T-47D). Methylated DNA was found in 6 cell lines (MCF7, SNU-1, MDA-MB-231, SK-BR-3, MCF-7 TH, and MCF 10A), and unmethylated DNA in 5 (MDA-MB-436, MDA-MB-468, SK-BR-3, ZR-75-1, and SNU-5). U and M denote unmethylation and methylation, respectively. N denotes normal human genomic DNA, which was used as an unmethylated PCR control, and DW is an abbreviation for distilled water, which was used as a negative PCR control.
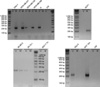
Fig. 5
Bicolor FISH analysis. Orange is produced by RUNX3 probe and green by control probe on 1q21.1. Ninety-five percent of ZR-75-1 cells showed a normal pattern (A), and O/G ratios of patterns were observed in all MCF7 cells (B).

Table 3
Results of bicolor FISH analysis
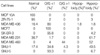
*Cell exhibited 2 pairs of orange and green colors; †The ratio of orange (representing the RUNX3 gene) to green (control) in the observed cell was less than 1; ‡The ratio of orange to green was more than 1; §Cell exhibited 2 pairs of orange and green colors; ∥Cell exhibited more than 2 pairs of orange and green colors.
FISH, fluorescent in situ hybridization.
ACKNOWLEDGMENT
We thank Kyu Choon Hwang, Ph.D (In2Gen Co., Seoul) for performing the MSP experiment and Pearl Lee (BMS Co., Seoul), Hankyu Seo (BMS Co., Seoul) for valuable discussions.
References
1. van Wijnen AJ, Stein GS, Gergen JP, Groner Y, Hiebert SW, Ito Y, Liu P, Neil JC, Ohki M, Speck N. Nomenclature for Runt-related (RUNX) proteins. Oncogene. 2004. 23:4209–4210.

2. Ito Y. Molecular basis of tissue-specific gene expression mediated by the runt domain transcription factor PEBP2/CBF. Genes Cells. 1999. 4:685–696.

3. Coffman JA. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int. 2003. 27:315–324.

4. Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer. 2005. 5:376–387.

5. Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002. 111:621–633.

6. Levanon D, Glusman G, Bangsow T, Ben-Asher E, Male DA, Avidan N, Bangsow C, Hattori M, Taylor TD, Taudien S, Blechschmidt K, Shimizu N, Rosenthal A, Sakaki Y, Lancet D, Groner Y. Architecture and anatomy of the genomic locus encoding the human leukemia-associated transcription factor RUNX1/AML1. Gene. 2001. 262:23–33.

7. Specchia G, Albano F, Anelli L, Zagaria A, Liso A, La Starza R, Mancini M, Sebastio L, Giugliano E, Saglio G, Liso V, Rocchi M. Insertions generating the 5'RUNX1/3'CBFA2T1 gene in acute myeloid leukemia cases show variable breakpoints. Genes Chromosomes Cancer. 2004. 41:86–91.

8. Osato M. Point mutations in the RUNX1/AML1 gene: another actor in RUNX leukemia. Oncogene. 2004. 23:4284–4296.

9. Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics. 1994. 23:425–432.

10. Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene. 2004. 23:4315–4329.

11. Zheng Q, Sebald E, Zhou G, Chen Y, Wilcox W, Lee B, Krakow D. Dysregulation of chondrogenesis in human cleidocranial dysplasia. Am J Hum Genet. 2005. 77:305–312.

12. Bae SC, Takahashi E, Zhang YW, Ogawa E, Shigesada K, Namba Y, Satake M, Ito Y. Cloning, mapping and expression of PEBP2 alpha C, a third gene encoding the mammalian Runt domain. Gene. 1995. 159:245–248.
13. Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002. 109:113–124.

14. Sakakura C, Hagiwara A, Miyagawa K, Nakashima S, Yoshikawa T, Kin S, Nakase Y, Ito K, Yamagishi H, Yazumi S, Chiba T, Ito Y. Frequent downregulation of the runt domain transcription factors RUNX1, RUNX3 and their cofactor CBFB in gastric cancer. Int J Cancer. 2005. 113:221–228.
15. Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW, Bang YJ, Kang GH. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004. 84:479–484.

16. Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, Le X, Yao J, Wu TT, Huang S, Xie K. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005. 65:4809–4816.

17. Oshimo Y, Oue N, Mitani Y, Nakayama H, Kitadai Y, Yoshida K, Ito Y, Chayama K, Yasui W. Frequent loss of RUNX3 expression by promoter hypermethylation in gastric carcinoma. Pathobiology. 2004. 71:137–143.
18. Ku JL, Kang SB, Shin YK, Kang HC, Hong SH, Kim IJ, Shin JH, Han IO, Park JG. Promoter hypermethylation downregulates RUNX3 gene expression in colorectal cancer cell lines. Oncogene. 2004. 23:6736–6742.

19. Goel A, Arnold CN, Tassone P, Chang DK, Niedzwiecki D, Dowell JM, Wasserman L, Compton C, Mayer RJ, Bertagnolli MM, Boland CR. Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers. Int J Cancer. 2004. 112:754–759.
20. Araki K, Osaki M, Nagahama Y, Hiramatsu T, Nakamura H, Ohgi S, Ito H. Expression of RUNX3 protein in human lung adenocarcinoma: implications for tumor progression and prognosis. Cancer Sci. 2005. 96:227–231.

21. Li QL, Kim HR, Kim WJ, Choi JK, Lee YH, Kim HM, Li LS, Kim H, Chang J, Ito Y, Youl Lee K, Bae SC. Transcriptional silencing of the RUNX3 gene by CpG hypermethylation is associated with lung cancer. Biochem Biophys Res Commun. 2004. 314:223–228.

22. Yanada M, Yaoi T, Shimada J, Sakakura C, Nishimura M, Ito K, Terauchi K, Nishiyama K, Itoh K, Fushiki S. Frequent hemizygous deletion at 1p36 and hypermethylation downregulate RUNX3 expression in human lung cancer cell lines. Oncol Rep. 2005. 14:817–822.

23. Schulmann K, Sterian A, Berki A, Yin J, Sato F, Xu Y, Olaru A, Wang S, Mori Y, Deacu E, Hamilton J, Kan T, Krasna MJ, Beer DG, Pepe MS, Abraham JM, Feng Z, Schmiegel W, Greenwald BD, Meltzer SJ. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett's-associated neoplastic progression and predicts progression risk. Oncogene. 2005. 24:4138–4148.

24. Mori T, Nomoto S, Koshikawa K, Fujii T, Sakai M, Nishikawa Y, Inoue S, Takeda S, Kaneko T, Nakao A. Decreased expression and frequent allelic inactivation of the RUNX3 gene at 1p36 in human hepatocellular carcinoma. Liver Int. 2005. 25:380–388.

25. Park WS, Cho YG, Kim CJ, Song JH, Lee YS, Kim SY, Nam SW, Lee SH, Yoo NJ, Lee JY. Hypermethylation of the RUNX3 gene in hepatocellular carcinoma. Exp Mol Med. 2005. 37:276–281.

26. Xiao WH, Liu WW. Hemizygous deletion and hypermethylation of RUNX3 gene in hepatocellular carcinoma. World J Gastroenterol. 2004. 10:376–380.

27. Li J, Kleeff J, Guweidhi A, Esposito I, Berberat PO, Giese T, Buchler MW, Friess H. RUNX3 expression in primary and metastatic pancreatic cancer. J Clin Pathol. 2004. 57:294–299.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download