Abstract
Thymidine phosphorylase (TP) has shown to be up-regulated in several cancers and to play a role in angiogenesis and invasion. Most studies regarding TP have focused on cancer cells. Recently, evidences suggest that TP in cancer-infiltrating inflammatory cells (CIICs) also affect the cancer cell behavior. To evaluate the significance of TP expression of CIICs in gastric cancer, we assessed TP expression of cancer cells and CIICs separately using immunohistochemical assay on 116 paraffin-embedded tissue samples from stomach cancer patients and investigated their clinical significance. When subjects were divided into 4 groups according to the TP expression: cancer/matrix (+/+), C/M (+/-), C/M (-/+), and C/M (-/-), intratumoral microvessel density scores were higher in the C/M (+/-) group than in the C/M (-/-) group (p=0.02). For lymph node metastasis and survival, there were no significant differences among the 4 groups. However, there were significant differences in survival (p=0.035) and LN metastasis (p=0.023) between the two groups divided by TP expression of CIICs alone irrespective of TP expression of cancer cells. Taken together, this study suggested the TP expression in CIICs could affect lymph node metastasis and patients' survival in gastric cancer.
Thymidine phosphorylase (TP) is an intracellular catalytic enzyme involved in the reversible conversion of thymidine to thymine. In addition, it has been reported that TP is completely identical to platelet-derived endothelial cell growth factor (PD-ECGF) and has angiogenic activity (1).
Compared with adjacent non-neoplastic tissue, higher levels of TP expression has been found in various cancer tissues (2). In the cancer tissue, high TP expression by enzyme-linked immunosorbent assay (ELISA) or positive TP expression on immunohistochemical staining have been found to be correlated with increased intratumoral microvessel density (IMVD) (3-5). Moreover, patients with high or positive TP expression in stomach cancer have shown a significantly worse outcome than patients with low or negative TP expression (6-11). For this reason, some investigators regard high or positive TP expression in stomach cancer as independent poor prognostic factors.
However, most of these studies have focused on TP expression in cancer cells alone or evaluated TP expression in cancer tissue without discriminating whether the TP comes from the cancer cells or cancer-infiltrating inflammatory cells (CIICs) (4, 6-11). Recently, It has been reported that much higher TP expression are observed in infiltrating-inflammatory cells in the interstitium around cancer cells rather than cancer cells themselves (7, 12-14). Although it has been reported that high TP expression in stomach cancer are observed in both CIICs and cancer cells themselves, little is known which TP plays more significant roles as a prognostic factor in the cancer tissue: TP in the cancer cells or in CIICs. In brain cancer, it was reported that CIICs, even though cancer cells themselves showed minimal TP expression, showed high expression of TP, and the high TP expression of CIICs was correlated with increased IMVD and poor prognosis (15).
We hypothesized the TP expression in CIICs has a strong impact on the biology of the stomach cancer and the patients' prognosis. To test this hypothesis, we separately examined TP expression in cancer cells and CIICs in stomach cancer to evaluate the significance of TP expression in CIICs in conjunction with various clinical parameters such as histological type, the depth of tumor invasion, lymph node metastasis, IMVD, and the patients' survival.
Paraffin-embedded tumor specimens were examined from 116 consecutively selected stomach cancer patients who had undergone surgery at the Gyeong-Sang National University Hospital from June 1998 to March 2002. The pathology reports and medical records were carefully reviewed to confirm the correct disease status. The age of patients (87 males and 29 females) ranged from 22 to 79 yr (median, 60 yr). The patients were followed up for 1 to 53 months (median, 27 months), and the follow-up period was regarded as the period after the surgery. After the initial review of all available hematoxilin and eosin (H&E)-stained slides of surgical specimens, we selected a representative paraffin block from each case for further study. The subjects were divided into four groups on the basis of the separate assessment of TP reactivity in cancer cells (cancer: C) and CIICs (matrix: M): cancer/matrix (+/+), C/M (+/-), C/M (-/+), and C/M (-/-). Of the 116 stomach cancer tissue samples, the subgroup of C/M (+/+), C/M (+/-), C/M (-/+), and C/M (-/-) were 38, 26, 39, and 13, respectively. We examined the correlation between the above mentioned patterns of TP expression and various parameters including IMVD, the depth of tumor invasion, lymph node metastasis, and patients' survival.
Sections were deparaffinized with xylene and dehydrated with 98% ethanol. The staining procedure was achieved by an immunoperoxidase technique on 5 µm-thick consecutive sections. Primary antibodies raised against TP (clone IC6-203, a generous gift from Nippon Roche research center, Japan, 1:750 dilution) and factor VIII (Dako, CA, U.S.A., 1:50 ) were used. For epitope retrieval, specimens were pretreated with microwaves and an extra trypsin treatment for factor VIII stain before incubation with primary antibodies (room temperature, 60 min) (15). The negative controls for some cases were parallel sections treated with the same process as above, but omitting the treatment of primary antibodies. Positive staining was visualized with diaminobenzidine, followed by a light hematoxylin counter staining.
Both staining intensity and percentage of positive cells were used to assess immunoreactivity of TP. The staining intensity was rated as weak, moderate, and strong as compared with the background stain or some negative controls. Tumors were considered as positive when more than 5% of cancer cells demonstrated moderate or strong staining intensity or more than 50% of cancer cells demonstrated weak intensity (16). The CIICs were considered as positive, when more than 50% of infiltrating mononuclear cells close to the cancer cells were stained with stronger than moderate intensity (14).
Histologic typing and grading of tumors were mainly performed according to the criteria established by the Japanese classification of gastric carcinoma (17).
For each case, IMVD was determined as the maximum number of microvessels under the light microscope within the "hot spot", which was defined as those areas containing the greatest number of capillaries and small venules at the invasive edge, based on the criteria of Weidner et al. (18). The "hot spot" was determined by scanning the tumor sections at low power (×40 and ×100). Subsequently, the microvessels were counted under the field using microscopic lens of ×200 power (×20 objective and ×10 ocular; 0.739 mm2/field) at the "hot spot". The evaluations were done by pathologists who were blinded to the patients' clinical status.
Among the 4 groups, the differences in both IMVD and the number of lymph nodes that contained metastatic tumor cells were examined using the Student's t-test. Any correlation between 4 groups and following clinical parameters such as N-stage, T-stage, histological type, age, and sex were assessed by chi-square test. The survival curve was calculated by the Kaplan-Meier method and then analyzed with the log-rank test. Statistical significance was defined as p<0.05.
TP expression was identified as being heterogeneous in the nucleus and/or the cytoplasm of cancer cells (Fig. 1). In most positive cases, the TP reactivity in CIICs was stronger than in cancer cells. Among the 4 subgroups, the cancer/matrix (+/+) pattern was the most frequent one in differentiated cancer, whereas cancer/matrix (-/+) was the most prevalent in the undifferentiated cancer (Table 1). Among the 4 subgroups, we could not find any significant difference in other clinical parameters such as age, sex, T-stage, and N-stage.
As shown in Fig. 2, the statistical differences in IMVD were observed among the 4 groups (p=0.02). Median IMVD score according to the TP staining pattern were 47 (range, 22-123), 54 (range, 22-123), 45.5 (range, 7-112), and 37 (range, 22-123) in the cancer (+)/matrix (+) group, cancer (+)/matrix (-), cancer (-)/matrix (+), cancer (-)/matrix (-), respectively. A statistically significant difference was found between cancer (+)/matrix (-) or cancer (+)/matrix (+) and cancer (-)/matrix (-) (p=0.02). This result suggests that TP expression in the cancer cells may affect on intratumoral microvessel formation.
There was no significant difference in the number of lymph node metastasis among the 4 groups. However, as shown in Fig. 3, the group with matrix (+), when we divided the subjects into two groups according to the TP reactivity of the CIICs irrespective of the TP reactivity of the cancer cells, showed to have a significantly larger number of metastatic lymph node than that with matrix (-) (p=0.019).
For the relationship between the TP expression pattern and patient's survival, the Kaplan-Meier analysis showed a trend of differences among the four groups (Fig. 4), but these differences, when assessed by a log-rank test, did not reach a significant value (p=0.089). However, there was a statistically significant difference in survival (p=0.02) when we assessed the matrix (+) or matrix (-) group with survival for the same relationship.
In this study, we found that high TP expression in CIICs in stomach cancer was correlated with advanced lymph node metastasis and poor prognosis. These findings are consistent with the evidence that infiltrating inflammatory cells adjacent cancer cells may affect tumor cell biology. Previous studies on TP expression demonstrated TP expression in cancer tissue is related to angiogenesis and poor prognosis, and they focused on the cancer cells themselves or evaluated TP expression without discriminating as to whether it came from cancer cells or CIICs (4, 6-11). One previous study demonstrated that much stronger TP expression was observed in CIICs than cancer cells in the stomach cancer and suggested that their TP expression should be related to angiogenesis and prognosis (9). However, it did not determine which TP is more important in angiogenesis or poor prognosis. The current study has demonstrated that high TP expression in CIICs in stomach cancer played more important roles in lymph node metastasis and patients' survival. The other merit of this study is that the separate assessment of TP expression leads to more accurate prediction of patients' prognosis and precise interpretation of other previous published results regarding TP expression in the stomach cancer. For example, two contradicting reports on TP expression are available, which showed a relationship between the histological type and TP expression in cancer tissue: one demonstrated that differentiated stomach cancer had a higher TP positive rate in tumor cell under immunohistochemical stain (9), whereas the other report, when measured by ELISA, revealed that the undifferentiated cancer type had higher TP levels in the stomach cancer tissue (8). According to our results, we found that the main source of TP in undifferentiated cancer tissue was CIICs (Fig. 1) and its TP activity was superior to that of cancer cells. Therefore, the contradicting results between the two studies might be assumed to have come from the results of the studies that measured TP from different main source: the former TP from cancer cells and the latter TP mainly from CIICs. Likewise, our separate interpretation can explain
the discrepancy of the two studies' results.
To assess the angiogenic activity, IMVD was measured; the relationship between the 4 TP expression patterns and IMVD was assessed in this study. The results demonstrated that the C/M (+/±) group had a higher IMVD than the C (-)/M (-) group, and this was consistent with previous studies (5, 7, 10, 11). In addition, the C/M (±/+) group seems to have higher IMVD than the group of C/M (-/-). Therefore, we divided the subjects into two groups by TP expression of cancer cells alone as well as CIICs alone to determine which factor plays more important role in angiogenesis. However, we could find no statistically significant differences between any two groups (data not shown). These results suggested that TP in CIICs might also play a cooperative role with TP in cancer cells for microvessel formation.
For survival, we found certain tendency of different effects among four groups, even though we did not clearly demonstrated the survival difference in each of the 4 group (C/M [+/+], C/M [+/-], C/M [-/+], and C/M [-/-]). This result suggested that TP expression in cancer cells also affect patients' prognosis, which is consistent with previous studies (3, 4). However, we could not find a statistically significant survival difference between two groups when we divided subjects by TP expression of cancer cells alone (data not shown). This finding suggests that the TP expression of cancer cells alone cannot predict the patients' prognosis. To prove the difference among the 4 groups in terms of prognosis, a further study on a much larger sample size seems to be needed. Before this study, based on a series of reports that had demonstrated that a high TP level in cancer tissue was related to poor prognosis (3, 7, 8, 12, 14, 20), we expected that the finding of TP expression of cancer cells in accordance with increased IMVD and lymph node metastasis could affect on the patients' survival. In contrast, our study revealed that TP expression in the CIICs have a stronger influence on lymph node metastasis than that of cancer cells, and the impact of TP expression of CIICs on microvessel formation may be similar to that of cancer cells. The finding that the survival was much influenced by TP expression in the CIICs in our study is in agreement with the study that reported that the TP-expressing macrophage in stomach cancer tissue may be an independent prognostic factor (10).
In summary, we performed separate interpretation of TP expression on cancer cells and CIICs to clarify the significance of each cell type. We found that the TP expression in CIICs may serve as an important variable that affects lymph node metastasis as well as new vessel formation, and ultimately, the survival of stomach cancer patients. These findings, may lead a change in interpreting the TP expression in stomach cancer tissue to predict the prognosis.
Figures and Tables
Fig. 1
Immunohistochemical staining for thymidine phosphorylase (TP) in a gastric cancer specimen using antibody against TP. TP expression was heterogeneously identified in nucleus and/or cytoplasm of cancer cells and cancer-infiltrating inflammatory cells. (A) Positive staining is observed in the cytoplasm and nucleus of cancer cells and CIICs, Cancer (+)/Matrix (+). (×200). (B) TP-reactivity is observed in the cytoplasm and nucleus of cancer cells and in less than 50% of CIICs, Cancer (+))/Matrix (-). (×200). (C) Note that most TP-positive cells were CIICs, Cancer (-)/CIICs (+). (×200). (D) TP-reactivity is observed in less than 50% of CIICs and less than 5% Cancer cells Cancer (-)/Matrix (-). (×400).
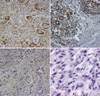
Fig. 2
Relationship between IMVD and TP expression. The statistical difference was found between cancer (-)/matrix (+) and cancer (-)/matrix (-) (p=0.02).

Fig. 3
Relationship between lymph node metastasis and TP expression. (A) No statistical difference in lymph node metastasis was shown among 4 groups according to TP expression. (B) The patients with positive TP expression in CIICs showed more lymph node metastasis in two groups divided by TP reactivity in CIIC (p=0.019).

Fig. 4
The Kaplan-Meier curves for survival according to the TP expression patterns. (A) The patients were divided into 4 groups according to TP expression. This graph shows a trend of survival differences among four groups, but these differences assessed by log-rank test did not reach a statistically significant value (p=0.089). (B) A clear survival difference was observed when the patients were divided into two groups as matrix-positive group and matrix-negative group regardless of TP reactivity of cancer cells (p=0.035).

ACKNOWLEDGMENTS
The authors thank Dr. Tae Sook Hwang at Inha University College of Medicine, Korea for her valuable technical advices. This study was supported by a grant from the Korean National Cancer Program, Ministry of Health & Welfare, R.O.K. and in part Korean Cancer Research Institute.
References
1. Focher F, Spadari S. Thymidine phosphorylase: a two-face Janus in anticancer chemotherapy. Curr Cancer Drug Targets. 2001. 1:141–153.

2. Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998. 34:1274–1281.

3. Tanigawa N, Amaya H, Matsumura M, Katoh Y, Kitaoka A, Aotake T, Shimomatsuya T, Rosenwasser OA, Iki M. Tumor angiogenesis and expression of thymidine phosphorylase/platelet derived endothelial cell growth factor in human gastric carcinoma. Cancer Lett. 1996. 108:281–290.
4. Sakatani T, Okamoto E, Tsujitani S, Ikeguchi M, Kaibara N, Ito H. Expressions of thymidine phosphorylase (dThdPase) and vascular endothelial growth factor on angiogenesis in intestinal-type gastric carcinoma. Oncol Rep. 2000. 7:831–836.

5. Osaki M, Sakatani T, Okamoto E, Goto E, Adachi H, Ito H. Thymidine phosphorylase expression results in a decrease in apoptosis and increase in intratumoral microvessel density in human gastric carcinomas. Virchows Arch. 2000. 437:31–36.

6. Konno S, Takebayashi Y, Aiba M, Akiyama S, Ogawa K. Clinicopathological and prognostic significance of thymidine phosphorylase and proliferating cell nuclear antigen in gastric carcinoma. Cancer Lett. 2001. 166:103–111.

7. Kimura H, Konishi K, Nukui T, Kaji M, Maeda K, Yabushita K, Tsuji M, Miwa A. Prognostic significance of expression of thymidine phosphorylase and vascular endothelial growth factor in human gastric carcinoma. J Surg Oncol. 2001. 76:31–36.

8. Kimura H, Konishi K, Kaji M, Maeda K, Yabushita K, Miwa A. Correlation between expression levels of thymidine phosphorylase (dThdPase) and clinical features in human gastric carcinoma. Hepatogastroenterology. 2002. 49:882–886.
9. Shimaoka S, Matsushita S, Nitanda T, Matsuda A, Nioh T, Suenaga T, Nishimata Y, Akiba S, Akiyama S, Nishimata H. The role of thymidine phosphorylase expression in the Invasivensess of gastric carcinoma. Cancer. 2000. 88:2220–2227.
10. Nagaoka H, Iino Y, Takei H, Morishita Y. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in macrophages correlates with tumor angiogenesis and prognosis in invasive breast cancer. Int J Oncol. 1998. 13:449–454.

11. Koukourakis MI, Giatromanolaki A, Kakolyris S, O'Byrne KJ, Apostolikas N, Skarlatos J, Gatter KC, Harris AL. Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non-small-cell lung cancer: impact on tumour neoangiogenesis and survival. Br J Cancer. 1998. 77:1696–1703.

12. Takahashi Y, Bucana CD, Akagi Y, Liu W, Cleary KR, Mai M, Ellis LM. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res. 1998. 4:429–434.
13. Yao Y, Kubota T, Sato K, Kitai R. Macrophage Infiltration-associated thymidine phosphorylase expression correlates with increased microvessel density and poor prognosis in astrocytic tumors. Clin Cancer Res. 2001. 7:4021–4026.
14. Giatromanolaki A, Koukourakis MI, Stathopoulos GP, Kapsoritakis A, Paspatis G, Kakolyris S, Sivridis E, Georgoulias V, Harris AL, Gatter KC. Angiogenic interactions of vascular endothelial growth factor, of thymidine phosphorylase, and of p53 protein expression in locally advanced gastric cancer. Oncol Res. 2000. 12:33–41.

15. Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996. 2:1679–1684.
16. Kono T, Nishida M, Inagaki N, Tanaka Y, Yoneda M, Kasai S. Development and characterization of 1C6-203, a new monoclonal antibody specific to human thymidine phosphorylase. J Histochem Cytochem. 2001. 49:131–138.

17. Japanese gastric cancer association. Japanese classification of gastric carcinoma, 2nd English Edition. Gastric Cancer. 1998. 1:10–24.
18. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast cancer. N Engl J Med. 1991. 324:1–8.
19. Shimaoka S, Matsushita S, Nitanda T, Matsuda A, Nioh T, Suenaga T, Nishimata Y, Akiba S, Akiyama S, Nishimata H. The role of thymidine phosphorylase expression in the Invasivensess of gastric carcinoma. Cancer. 2000. 88:2220–2227.
20. Koizumi W, Saigenji K, Nakamaru N, Okayasu I, Kurihara M. Prediction of response to 5'-deoxy-5-fluorouridine (5'-DFUR) in patients with inoperable advanced gastric cancer by immunostaining of thymidine phosphorylase/platelet-derived endothelial cell growth factor. Oncology. 1999. 56:215–222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download