Abstract
Marchiafava-Bignami disease (MBD) is a rare alcohol-related disorder that results in progressive demyelination and necrosis of the corpus callosum. The process may extend to the optic chiasm and tracts, cerebellar peduncle, subcortical resion, neighboring white matter, and rarely, cortical gray matter. We report a case of MBD in which fluid-attenuated inversion recovery and diffusion magnetic resonance imaging studies revealed symmetrical hyperintense lesions in the cerebral cortex in addition to the callosal lesions.
One of the most important pathologic findings in Marchiafava-Bignami disease (MBD) is the symmetrical demyelination of the middle portion of the corpus callosum, observed in patients with chronic alcoholism (1). The clinical diagnosis of MBD can be difficult. The computerized tomography (CT) and magnetic resonance imaging (MRI) are helpful in the detailed analysis of the distribution of lesions as well as establishing the diagnosis (2). Recent MRI studies have shown that the lesions may also be found in the hemispheric white matter or cortical gray matter (2, 3). We report a case of acute MBD with widespread callosal and cortical lesions.
A 49-yr-old man was admitted to our hospital because of acute onset of seizures and altered mental status. He had abused alcohol for 10 yr. On examination, the patient was stuporous; however, he showed no lateralizing signs. Blood and cerebrospinal fluid studies were within normal limits. T2-weighted images (T2WI) and diffusion-weighted images (DWI) obtained on admission demonstrated diffuse hyperintense lesions involving the splenium and the body of the corpus callosum (Fig. 1). In addition, symmetric cerebral cortical hyperintense lesions were observed mainly in the frontal regions (Fig. 1).
On the basis of clinical history and imaging features, the diagnosis of MBD was made, and high-dose vitamin B complex including 1,000 mg/day thiamine and corticosteroid were administered intravenously for three weeks. However, the patient remained in a persistent stuporous mental status. Follow-up axial T2WI and DWI studies, 15 days after the initial study, showed diminished signal intensity in bilateral cortical lesions and in the corpus callosum; there were focal areas of hypointensity in the genu and splenium. Twelve weeks after the initial study, there was severe atrophy of the corpus callosum with multiple focal areas of presumed necrosis in the genu, body, and splenium (Fig. 2).
Although spontaneous verbal production and repetition were absent, he could understand only simple spoken commands.
MBD results in acute demyelination and necrosis of the corpus callosum; it is associated with chronic alcohol consumption but is occasionally seen in non-alcoholic patients. It is generally accepted that the disease is mainly due to the deficiency of the vitamin B complex (1, 2).
The disease may present in two major clinical forms: acute and chronic. In the chronic form, an interhemispheric disconnection syndrome, such as limb apraxia, tactile agraphia, unilateral agraphia, hemialexia, and dementia, can be seen and can last for several months to several years. The acute form with severe impairment of consciousness, seizures, and muscle rigidity often results in death after several days.
Clinicians can confuse MBD with Wernicke's encephalopathy (WE), and some reports have shown that MBD and WE often occur concurrently (2). Our patient did not have WE, since he did not have any neurological abnormalities such as ophthalamoplegia, and a prominent corpus callosal lesion was seen in the absence of abnormal MRI findings at the medial thalamus, mammillary body, and periaqueductal brain stem, which are usually found in patients with WE.
The diagnosis of MBD is based on the callosal lesions. Differentiation of MBD from infarction of the corpus callosum or multiple sclerosis may be difficult. However, selective involvement of the entire length of the corpus callosum and focal cystic necrosis confined to its central layer are more likely to be due to MBD (4, 5). The combination of chronic alcoholism, other clinical features, and MRI findings support the correct diagnosis.
In patients with MBD, extracallosal lesions, such as hemispheric white matter and middle cerebellar peduncle, are not rare (5-7). A highly unusual feature in our patient was the diffuse cortical involvement, especially frontal cortical lesions. Postmortem neuropathologic study in patients with MBD has revealed a type of cerebral cortical lesion. This cortical lesion, known as Morel's laminar sclerosis, mainly in the third layer and especially in the lateral-frontal cortex, is associated with, and probably secondary to, the callosal lesions of MBD (2). A possible explanation for the co-existence of cortical and callosal lesions in our patient was the central pontine (CPM) and extrapontine myelinolysis (EPM), following a marked change in osmolarity, due to rapid correction of hyponatremia, hypernatremia, malnutrition, malignancy, and alcoholism. According to a previous report, MBD resembles CPM and EPM in its histological features as well as its symmetry and central location. For these reasons the two disorders are often discussed together. A variety of sites may be involved in EPM. Cerebellum, external capsule, putamen, thalamus, and cerebral cortex are common sites for lesions with EPM (8-10). However, most cases of EPM spare the corpus callosum. This fact tends to negate any relationship between the two diseases. On the other hand, McComb reported a patient diagnosed as CPM and EPM, and on a postmortem study, symmetric demyelination was found at the pontine, thalamus, cerebellum as well as the corpus callosumg. This report supports that the corpus callosum is a probable site for lesions of EPM. If this assumption is correct, EPM may have been the possible mechanism causing simultaneous callosal and cortical lesions in our patient. However, further studies are necessary to delineate the pathologic details of MBD.
We report an unusual case of MBD where the MRI showed acute cortical abnormalities in addition to callosal lesions. In a patient with these MRI findings, typical history and clinical manifestations, the possibility of MBD should be considered.
Figures and Tables
Fig. 1
Diffusion weighted images (A, B) and T2-weighted image (C) show bright, high signal intensities in the corpus callosum and bilateral frontal cortex.
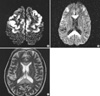
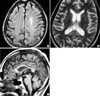
Fig. 2
Follow-up Fluid attenuated inversion recovery and T2-weighted images after months show decreased high signal intensities of bilateral frontal cortex and corpus callosum (A, B). Sagittal T1-weighted image reveals atrophy of the corpus callosum with multiple focal areas of presumed necrosis in the genu, body, and splenium (C).
References
1. Ferracci F, Conte F, Gentile M, Candeago R, Foscolo L, Bendini M, Fassetta G. Marchiafava-Bignami disease: computed tomographic scan, 99mTc HMPAO-SPECT, and FLAIR MRI findings in a patient with subcortical aphasia, alexia, bilateral agraphia, and left-handed deficit of constructional ability. Arch Neurol. 1999. 56:107–110.
2. Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol. 2005. 26:670–673.
3. Ménégon P, Sibon I, Pachai C, Orgogozo JM, Dousset V. Marchiafava-Bignami disease: Diffusion weighted MRI in corpus callosum and cortical lesions. Neurology. 2005. 65:475–477.
4. Chang KH, Cha SH, Han MH, Park SH, Nah DL, Hong JH. Marchiafava-Bignami disease: serial changes in corpus callosum on MRI. Neuroradiology. 1992. 34:480–482.

5. Friese SA, Bitzer M, Freudenstein D, Voigt K, Küker W. Classification of acquired lesions of the corpus callosum with MRI. Neuroradiology. 2000. 42:795–802.

6. Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol. 2003. 24:1955–1957.
8. Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004. 75:Suppl 3. 22–28.

9. McComb RD, Pfeiffer RF, Casey JH, Wolcott G, Till DJ. Lateral pontine and extrapontine myelinolysis associated with hypernatremia and hyperglycemia. Clin Neuropathol. 1989. 8:284–288.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download