Abstract
Ceramides are the main lipid component maintaining the lamellae structure of stratum corneum, as well as lipid second messengers for the regulation of cellular proliferation and/or apoptosis. In our previous study, psoriatic skin lesions showed marked decreased levels of ceramides and signaling molecules, specially protein kinase C-alpha (PKC-α) and c-jun N-terminal kinase (JNK) in proportion to the psoriasis area and severity index (PASI) scores, which suggested that the depletion of ceramide is responsible for epidermal hyperproliferation of psoriasis via downregulation of proapoptotic signal cascade such as PKC-α and JNK. In this study, we investigated the protein expression of serine palmitoyltransferase (SPT) and ceramidase, two major ceramide metabolizing enzymes, in both psoriatic epidermis and non-lesional epidermis. The expression of SPT, the ceramide generating enzyme in the de novo synthesis in psoriatic epidermis, was significantly less than that of the non-lesional epidermis, which was inversely correlated with PASI score. However, the expression of ceramidase, the degradative enzyme of ceramides, showed no significant difference between the lesional epidermis and the non-lesional epidermis of psoriatic patients. This might suggest that decreased expression of SPT protein is one of the important causative factors for decreased ceramide levels in psoriasis.
Ceramides are the main lipids in the stratum corneum, and their depletion is thought to be one of the etiological factors for barrier disruption in various levels of skin conditions (1-4). Marked depletion of ceramides in the stratum corneum has been reported especially in patients with psoriasis and atopic dermatitis.
Ceramides are generated by serine palmitoyltransferase (SPT), the enzyme for de novo synthesis or by the action of sphingomyelinase. As lipid second messengers, they mediate antiproliferative and apoptotic effect via activation of several signal transduction molecules such as protein kinase C-alpha (PKC-α) and c-jun N-terminal kinase (JNK) (5-7). In our previous study, the decreased levels of ceramide in psoriatic skin lesions were shown to be responsible for epidermal hyperplasa via downregulation of proapoptotic signal cascade such as PKC-α and JNK. In contrast, diacyglycerol (DAG) levels were elevated and free fatty acid levels were not changed (8, 9). However, in the past study, the ceramide level and protein expression of ceramide-metabolizing enzymes in psoriatic skin lesion was not elucidated. In the present study measured the expression of SPT, key enzyme for de novo ceramide synthesis, and ceramidase, the degradative enzyme of ceramide, in both psoriatic epidermis and non-lesional epidermis.
Twenty four Korean patients with psoriasis (9 women, 15 men) ranging in age from 9 to 37 yr gave informed consent and took part in this study. All the subjects had psoriasis vulgaris as identified through clinical and histological assessment and had not been treated either systemically or topically for at least 1 month before punch biopsies were done (Table 1). Using a 4-mm punch, biopsies were taken from lesional and non-lesional skin on the lower extremities, back, or arms. The epidermis was separated as described previously (10). Specifically, the epidermis was separated from whole-skin biopsies by overnight incubation at 4℃ in a 1:1 (v/v) mixture of dispase solution (Roche Molecular Biochemicals, Manheim, Germany) and Hank's balanced salt solution (HBSS; Gibco BRL, Life Technologies, Rockville, MD, U.S.A.).
The clinical severity was assessed using the psoriasis area and severity index (PASI) score, which is calculated as follows: PASI=0.1 (Eh+Ih+Dh)Ah+0.2 (Eu+Iu+Du)Au+0.3 (Et+It+Dt)At+0.4 (El+Il+Dl)Al, where E=erythema, I=infiltration, D=desquamation, A=area, h=head, u=upper extremities, t=trunk, and l=lower extremities. A numerical value is given to the extent of the lesions in each area: 1=<10%, 2=10-30%, 3=30-50%, 4=50-70%, 5=70-90%, and 6=90-100%. E, I, and D were scored on a five-point scale (0=no symptoms, 1=slight, 2=moderate, 3=marked, and 4=very marked) to obtain a final PASI score between 0 and 72. The PASI scores of the patients who took part in this study ranged between 2.0 and 27.4. The patients with PASI scores <30 were enrolled in this study in order to determine whether alterations in the levels of SPT and ceramidase are closely correlated to the clinical severity in psoriatic patients (Table 1). All patients with PASI scores >30 were excluded in this study because their skin lesions were shown to be transformed into severe variants of psoriasis, such as the erythrodermic type.
Every frozen skin sample was added to 400 µL of Folch solution (CHCl3: MeOH 2:1, v/v mixture) and was homogenized using a polytron homogenizer, and 200 µL of 0.1 M KCl was added. The mixture was centrifuged at 3,000 rpm for 5 min each. The upper phase containing the extracted proteins was separated. This process was repeated one more time. The protein concentration was determined by BCA protein assay (Pierce, Rockford, U.S.A.) according to the manufacturer's protocol. Fifty micrograms of protein was fractionated on 10% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane by electrophoresis. The membranes were blocked with 5% non-fat dry milk for 1 hr at room temperature and incubated with prepared human SPT, ceramidase, and tubulin antibody (Labfrontier, Seoul, Korea) at a 1:250 dilution with Tris-buffered saline containing 0.05% Tween-20 (TSB-T) for 1 hr. After washing with TBS-T for 1 hr, the membranes were reacted with horseradish peroxidase-conjugated anti-rabbit IgG antibody (Amersham Biosciences, Buckinghamshire, U.K.) diluted 1:250 with TBS-T for 1 hr at room temperature. After washing the membranes with TBS-T for 1 hr, the protein level was detected using the ECL chemiluminescence kit (Amersham Bioscience, Buckinghamshire, U.K.).
The statistical significance of the difference in the levels of SPT/tubulin and ceramidase/tubulin between the lesional and the non-lesional samples of psoriasis patients was assessed using a paired t-test. The correlations between the levels of SPT and the PASI scores were determined using the Spearman correction test. p values less than 0.05 were considered statistically significant.
Western blot analysis of SPT and ceramidase in the lesional and non-lesional epidermis of psoriasis patients is shown in Fig. 1, 2. When the level of SPT protein expression was quantified by densitometry and was expressed as a ratio of SPT to tubulin (Fig. 1, 2), the levels of SPT expression in the lesional epidermis was significantly lower (range, 1.26-5.59) than those in the non-lesional epidermis ( range, 4.63-9.97) (Fig. 1). In contrast, levels of ceramidase expression in the lesional epidermis ranged from 0.025 to 1.070, and those in the non-lesional epidermis ranged from 0.023 to 1.556, which indicates that there was no significant difference in the level of ceramidase of lesional and non-lesional epidermis of psoriasis (Fig. 2). After normalization with tubulin, the mean level of SPT/tubuin in the lesional epidermis was significantly decreased compared to the non-lesional epidermis as shown in Fig. 3A (p<0.05). However, there was no significant difference in the mean ratio of ceramidase to tubulin between lesional psoriatic skin and non-lesional skin (Fig. 3B).
The protein expression of SPT and ceramidase of non-lesion and lesion, and the PASI score in psoriatic patients are shown in Table 1. Although the absolute level of SPT varied from patient to patient, SPT expression of lesional epidermis was significantly less (p<0.05) than that of non-lesional epidermis, as with the percent reduction ranging from 17.5 to 87.6. When the PASI score was applied, there was a significant negative correlation between percent reduction of SPT expression in the lesional epidermis and the PASI score (r=-0.76, p=0.01) as shown in Fig. 4. However, there was no significant correlation between percent reduction of ceramidase expression in the lesional epidermis and the PASI score.
The epidermis contains a large amount of sphingolipids, which are concentrated in upper, differentiated cell layer, where they are thought to participate in the maintenance of the cutaneous permeability barrier (11). Among the epidermal sphingolipids, ceramide is the main lipid in the stratum corneum and its depletion is thought to be one of the etiological factors for barrier disruption in skin conditions (1-4). Moreover, as a signal transduction molecule, ceramide is involved in the cell proliferation and apoptosis signaling pathway via activation of several signal transduction molecules, and its depletion is related with various skin disease such as atopic dermatitis or psoriasis (5-8). Synthesis of ceramide is regulated by several enzymes such as sphingomyelinase, β-glucocerebrosidase, and ceramidase (12-15). Also a de novo pathway mediated by SPT functions as a key pathway of ceramide systhesis (16) (Fig. 5).
SPT is a key enzyme in sphingolipid biosynthesisis. It is the first committed and rate-limiting step in sphigolipid synthesis (17, 18). One of the substrates for SPT is various acyl-CoAs. In the mammalian cells, palmitoyl CoA is one of the most abundant acly-CoA types and it is the predominant acyl-CoA substrate of SPT in vivo (19-21). However, SPT strictly utilizes L-serine as its amino acid substate (22). In human epidermis, SPT catalyzes the palmitoyl CoA and L-serine to synthesize ceramide (17).
Ceramidase hydrolyzes ceramide into shpingophosphorylcholine and free fatty acid. It is demonstrated that marked elevated activity of this enzyme is responsible for low levels of cermaide in stratum corneum of atopic dermatitis patients (23).
In our previous study, decreased ceramide in psoriatic skin lesions is shown to be responsible for epidermal hyperplasia via downregulation of proapoptotic signal cascade such as PKC-α and JNK. In contrast, DAG levels were elevated and free fatty acid levels were not changed (8, 9). However, in the past study, we could not elucidate what makes ceramide decreased in psoriatic skin lesion.
In this study, we measured the expression of SPT and ceramidase in both psoriatic epidermis and non-lesional epidermis. SPT is one of the enzymes, which is related to the synthesis of ceramide, and ceramidase is a catalytic enzyme, which is related to the decrease of the levels of ceramide. The results of this experiment showed that levels of SPT were significantly decreased in the psoriatic skin. However, the levels of ceramidase showed no significant difference between psoriatic epidermis and non-lesional epidermis. We also evaluated the relationship between the PASI score and decreased levels of SPT to determine the role of decreased SPT on the severity of psoriasis. The correlation between the percentage reduction in the ratio of SPT/tubulin in the lesional epidermis and the PASI scores showed a significantly negative correlation. Considering above results, decreased levels of ceramide in psoriatic skin, as shown in our previous study, are responsible for decreased levels of SPT in psoriatic skin lesion, and their levels of reduction are highly related to the clinical severity of psoriasis. However, according to this experiment, ceramidase does not seem to have a relation with psoriatic epidermal changes, and this result is different from that of the previous study on ceramidase activity in atopic dermatitis (23).
In conclusion, we demonstrated that the decreased levels of ceramide in the psoriatic epidermis is related to its de novo synthetic pathway, which is associated with levels of SPT, and the levels of SPT are closely related to the clinical severity of psoriasis. Considering that psoriasis is one of the inflammatory skin diseases, several cytokines or growth factors secreted by keratinocytes, Langerhans cells, or T-cells in the psoriatic skin lesion seem to affect the expression of serine palmitoyltransferase, and this postulation need further studies.
Figures and Tables
 | Fig. 1Western blot analysis of SPT in the lesional and the non-lesional epidermis of posriasis patients. Western blotting was performed using anti-SPT antibodies, as described in Materials and Method. The level of SPT expression was quantified by densitometry and is expressed as ratio of SPT and tubulin. *, significant differences between lesional epidermis and non-lesional epidermis (p<0.05). |
 | Fig. 2Western blot analysis of ceramidase in the lesional and the non-lesional epidermis of posriasis patients. Western blotting was performed using anti-ceramide antibodies, as described in the Materials and Method. The level of ceramidase expression was quantified by densitometry and is expressed as a ratio of ceramidase and tubulin. However, we could not find any statistically significant difference between two groups. |
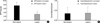 | Fig. 3(A) The mean ratio of SPT and tubulin of the lesional epidermis was significantly decreased compared to that of the non-lesional epidermis. *, significant differences between lesional epidermis and non-lesional epidermis (p<0.05). (B) However, there was no significant difference between the mean ratio of ceramidase to tubulin in the lesional epidermis and that of the non-lesional epidermis. |
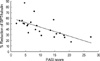 | Fig. 4Correlation between the percentage reduction in the ration of SPT/tubulin in the lesional epidermis and the PASI scores of psoriasis patients. A significant negative correlation was observed (Spearman's rho=-0.76, p=0.01). |
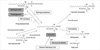 | Fig. 5The synthesis of ceramide is regulated by several enzymes such as sphingomyelinase, β-glucocerebrosidase, and ceramidase. Also a de novo pathway mediated by serine palmitoyltransferase (SPT) functions as the key pathway of ceramide systhesis. SM, spingomyelin; SPT, serine palmitoyltransferase. |
Table 1
SPT, ceramidase, and PASI score

*Significant difference of SPT/tubulin levels between the lesional epidermis and non-lesional epidermis (n=24, p<0.05). †The percent reduction was calculated as (SPT/tubulin in lesion divided by SPT/tubulin in non-lesional level)×100 . ‡The percent reduction or increase was calculated as (ceramidase/tubulin in lesion divided by ceramidase/tubulin in non-lesional level)×100. S, serine palmitoyltransferase; T, tubulin; C, ceramidase.
References
1. Elias PM, Menon G. Elias PM, editor. Structural and lipid biochemical correlates of the epidermal permeability barrier. Skin Lipid. Advances in Lipid Research. 1999. 24. San Diego: Academic Press;26.

2. Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased levels of ceramides in stratum corneum of atopic dermatitis: an etiological factor in atopic dry skin. J Invest Dermatol. 1991. 96:523–526.
3. Matsumoto M, Umemoto N, Sugiura H, Uehara M. Difference in ceramide composition between "dry" and normal skin in patients with atopic dermatitis. Acta Derm Venereol (Stockh). 1999. 79:246–247.
4. Chung S, Kong S, Seong K, Cho Y. γ-Linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs. J Nutr. 2002. 132:3090–3097.

5. Aschrafi A, Franzen R, Shabahang S, Fabbro D, Pfeilschifter J, Huwiler A. Ceramide induces translocation of protein kinase C-alpha to the Golgi compartment of human embryonic kidney cells by interacting with the C2 domain. Biochim Biophys Acta. 2003. 1634:30–39.
6. Huwiler A, Fabbro D, Pfeilschifter J. Selective ceramide binding to protein kinase C-alpha and -delta isoenzymes in renal mesangial cells. Biochemistry. 1998. 37:14556–14562.
7. Ruvolo PP. Ceramide regulates cellular homeostasis via diverse stress signaling pathways. Leukemia. 2001. 15:1153–1160.

8. Cho Y, Lew BL, Seong K, Kim NI. An inverse relationship between ceramide synthesis and clinical severity in patients with psoriasis. J Korean Med Sci. 2004. 19:859–863.

9. Lew BL, Cho Y, Kim J, Sim WY, Kim NI. Ceramides and cell signaling molecules in psoriatic epidermis: reduced levels of ceramides, PKC-alpha, and JNK. J Korean Med Sci. 2006. 21:95–99.
10. Macheleidt O, Kaiser HW, Sandhoff K. Deficiency of epidermal protein-bound ω-hydroxyceramides in atopic dermatitis. J Invest Dermatol. 2002. 119:166–173.

11. Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983. 24:120–130.

12. Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000. 10:73–80.

13. Perry DK. The role of de novo ceramide synthesis in chemotherapy-induced apoptosis. Ann N Y Acad Sci. 2000. 905:91–96.

14. Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000. 1485:63–99.

15. van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J. 2003. 369:199–211.
16. Merrill AH Jr, Jones DD. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990. 1044:1–12.

17. Holleran WM, Williams ML, Gao WN, Elias PM. Serine palmitoyltransferase activity in cultured human keratinocytes. J Lipid Res. 1990. 31:1655–1661.
18. Holleran WM, Gao WN, Feingold KR, Elias PM. Localization of epidermal sphingolipid synthesis and serine palmitoyltransferase activity: alterations imposed by permeability barrier requirements. Arch Dermatol Res. 1995. 287:254–258.
19. Tardi PG, Mukherjee JJ, Choy PC. The quantitation of long-chain acyl-CoA in mammalian tissue. Lipids. 1992. 27:65–67.

20. Mangino MJ, Zografakis J, Murphy MK, Anderson CB. Improved and simplified tissue extraction method for quantitating long-chain acyl-coenzyme A thioesters with picomolar detection using high-performance liquid chromatography. J Chromatogr. 1992. 577:157–162.

21. DeMar JC Jr, Anderson RE. Identification and quantitation of the fatty acids composing the CoA ester pool of bovine retina, heart, and liver. J Biol Chem. 1997. 272:31362–31368.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download