Abstract
The house dust mite (HDM) is considered to be the most common indoor allergen associated with bronchial asthma. In this study, we investigated whether crude extract of the HDM Dermatophagoides farinae could activate human eosinophilic leukemic cells (EoL-1) to induce upregulation of cell-surface adhesion molecules. When EoL-1 cells were incubated with D. farinae extract, expression of intercellular adhesion molecule-1 (ICAM-1) significantly increased on the cell surfaces compared to cells incubated with medium alone. In contrast, surface expression of CD11b and CD49d in EoL-1 cells was not affected by D. farinae extract. In addition, pretreatment of cells with NF-κB inhibitor (MG-132) or JNK inhibitor (SP600125) significantly inhibited ICAM-1 expression promoted by HDM extract. However, neither p38 MAP kinase inhibitor nor MEK inhibitor prevented HDM-induced ICAM-1 expression in EoL-1 cells. These results suggest that crude extract of D. farinae induces ICAM-1 expression in EoL-1 cells through signaling pathways involving both NF-κB and JNK.
Eosinophils are major inflammatory effector cells, and blood and tissue eosinophilia is a feature of many allergic disorders, such as bronchial asthma (1). The mechanisms controlling eosinophil-mediated tissue inflammation involve the regulation of chemoattractant-dependent interactions with the vascular endothelium and intercellular or cell-extracellular matrix interactions (2). Such interactions are complex processes largely dependent on the expression of adhesion molecules on the eosinophil cell membrane. It has been demonstrated that intercellular adhesion molecule-1 (ICAM-1) is an important factor in many allergic diseases such as asthma (3). ICAM-1 belongs to the immunoglobulin (Ig) superfamily of cellular adhesion molecules and can bind to lymphocyte function-associated antigen-1 (LFA-1), macrophage antigen-1 (Mac-1), fibrinogen, hyaluronan, and CD43 on leucocytes (4). The upregulation of ICAM-1 expression has been reported in activated eosinophils in response to cytokines such as interleukin-3 (IL-3), IL-5, granulocyte macrophage colonystimulating factor (GM-CSF), and IL-25 (5, 6). Therefore, enhanced expression of ICAM-1 in activated eosinophils at the site of allergic inflammation may allow cell contact-dependent regulation of immune cells in bronchial asthma.
The house dust mite (HDM) Dermatophagoides is the most common indoor allergen and a major cause of perennial asthma worldwide (7). In vitro studies suggest that HDM allergens directly affect a variety of cell types including bronchial epithelial cells and T cells (8, 9), leading to amplification of allergen-induced bronchial asthma. In addition, it has been reported that extract of HDM D. pteronyssinus can activate eosinophils to generate GM-CSF, TNF-α, and IL-8 by activation of NF-κB (10). The transcription factor NF-κB is a central regulator of the immune system and promotes the transcription of over 150 genes (11). For example, upon stimulation with pro-inflammatory cytokines such as TNFα or IL-1β, IκB kinase is activated to phosphorylate IκB, which is then degraded by the proteasome. Degradation of IκB allows NF-κB to translocate into the nucleus where it can bind to κB sequences in the promoters of NF-κB-dependent genes to upregulate transcription. Recent studies have reported that activation of NF-κB mediates the transcription of ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and cytokines such as GM-CSF and IL-8 in leukocytes (10, 12). However, no information is available regarding the direct effect of D. farinae extract on the expression of adhesion molecules in human eosinophils.
Human eosinophilic leukemic cells (EoL-1) are a useful in vitro model for studying the functions and regulation of human eosinophils. D. farinae has been shown to be a predominant species of HDM in Korea (13). Therefore, in the present study, we investigated the effect of D. farinae extract on ICAM-1 expression on the surfaces of EoL-1 cells, and the involvement of NF-κB in the expression of ICAM-1 induced by D. farinae extract.
The human eosinophic leukemic cell line EoL-1 (ECACC 94042252) was maintained in RPMI 1640 medium with 25 mM HEPES (Gibco Laboratories, Grand Island, NY, U.S.A.) supplemented with 10% heat inactivated fetal bovine serum (FBS, Gibco Laboratories) in 5% CO2 and 95% humidified air at 37℃. Pharmacologic inhibitors used in this study were MG-132 (NF-κB proteasome inhibitor), SB-203580 (p38 MAPK inhibitor), U0126 (MEK 1/2 inhibitor), and SP600125 (JNK inhibitor II), which were purchased from Calbiochem Corp (San Diego, CA, U.S.A.) and Cell Signaling (Beverly, MA, U.S.A.). EoL-1 cells were pretreated with various inhibitors for 1 hr at 37℃ before incubation with HDM extract.
Live or frozen D. farinae, reared at the Department of Parasitology, Yonsei University College of Medicine, were pulverized in liquid nitrogen. The HDM sample was defatted with ethylether and then extracted in 100 mL phosphate-buffered saline (PBS) (137 mM NaCl, 1.8 mM KH2PO4, 10 mM Na2HPO4, 27 mM KCl, pH 7.4) for 72 hr at 4℃ under constant stirring. The extract was centrifuged at 10,000 g for 1 hr at 4℃, and the resulting supernatant was dialyzed (cutoff molecular weight 1 kDa; Spectrum, Houston, TX, U.S.A.) against distilled water for 48 hr. The dialyzed supernatant was lyophilized and stored at -20℃ until use. Endotoxin, measured by the E-toxate kit (Sigma Chemical Co., St. Louis, MO, U.S.A.), was not detected in the HDM extract sample. The kit was sensitive to 0.05-0.1 endotoxin units/mL. The amount of total protein in the extract was measured by a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, U.S.A.).
EoL-1 cells (1×106/mL) were incubated for 1 hr in 48-well flat-bottom tissue culture plates (Costar, Cambridge, MA, U.S.A.) with or without MG-132, SB203580, U0126 or SP600125 at 37℃ in a 5% CO2 incubator. Following this preincubation, the cells were incubated in the presence or absence of HDM extract (5-200 µg/mL) for 4.5 hr in a 5% CO2 incubator. TNF-α (20 ng/mL) (R & D systems, Minneapolis, MN, U.S.A.) was used as a positive control. After incubation, the cells were washed with cold PBS containing 1% FBS and then incubated for 30 min at 4℃ in dark with FITC-conjugated mouse anti-human ICAM-1 mAb (BD Pharmingen, San Diego, CA, U.S.A.), PE-conjugated mouse anti-human CD49d (BD Pharmingen) or PE-labeled mouse anti-human CD11b mAb (BD Pharmingen). FITC- or PE-conjugated mouse IgG1 (BD Pharmingen) were used as isotype control antibodies. After washing, the cells were resuspended in 200 µL of PBS buffer for flow cytometric analysis. Flow cytometric analysis for fluorescent intensity of ICAM-1, CD49d or CD11b expression on EoL-1 cells was performed on at least 10,000 cells from each sample with the FACScan instrument (BD Biosciences, San Diego, CA, U.S.A.).
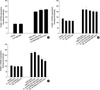
To address whether extract of D. farinae is capable of inducing surface expression of ICAM-1 in EoL-1 cells, we incubated EoL-1 cells with various concentrations (5-200 µg/mL) of HDM extract for 4.5 hr. As shown in Fig. 1, HDM extract induced ICAM-1 expression in EoL-1 cells in a dose-dependent fashion. At 25, 50, 100, and 200 µg/mL, HDM extract strongly induced ICAM-1 expression (mean±SEM fluorescent intensity: 195.1±5.8, 221.7±7.4, 241.6±9.3, 255.8±12.2, respectively). TNF-α (20 ng/mL) also significantly increased the fluorescent intensity of ICAM-1 expression on EoL-1 cells (mean±SEM fluorescent intensity: medium 137.6±6.3; TNF-α treatment 249.9±13.0).
Next, we investigated the effect of HDM extract on the expression of CD11b or CD49d in EoL-1 cells. Treatment of EoL-1 cells with HDM extract (100 µg/mL) for 4.5 hr did not cause a significant increase in CD11b or CD49d expression compared to cells incubated in medium alone (data not shown).
ICAM expression is highly NF-κB-dependent (14), and so we investigated the inhibitory effect of NF-κB inhibitor MG-132 on HDM extract-induced upregulation of ICAM-1 in EoL-1 cells. As shown in Fig. 2, pretreatment with 5 µM MG-132 resulted in the complete reduction of ICAM expression induced by HDM extract. No cytototoxic effect of MG-132 alone at the concentration tested was observed.
A recent report demonstrated that MAPKs, including ERK1/2 and JNK, regulate ICAM-1 expression due to stimulation by cytokines (15). We examined the role of MAPKs in HDM extract-induced ICAM-1 expression in EoL-1 cells by using p38 MAPK inhibitor (SB203580), MEK 1/2 inhibitor (U0126), or JNK inhibitor (SP600125). As shown in Fig. 3A, B, pretreatment with SB203580 (10 µM) or U0126 (10-100 µM) did not inhibit HDM extract-triggered upregulation of ICAM expression in EoL-1 cells. In contrast, as shown in Fig. 3C, JNK inhibitor SP600125 reduced expression of ICAM-1 by HDM extract in a dose-dependent manner.
In the present study, we have demonstrated that crude extract of HDM D. farinae activates EoL-1 cells to induce ICAM-1 expression. Untreated EoL-1 cells expressed low levels of ICAM-1, but HDM extract significantly enhanced the cell-surface expression of ICAM-1 in a dose-dependent manner. In addition, inhibition of NF-κB with the proteosome inhibitor MG-132 almost completely prevented the surface expression of ICAM-1 in EoL-1 cells induced by HDM extract, suggesting a critical role of NF-κB in the expression of ICAM-1. This result is in line with previous reports (5, 16) that ICAM-1 expression promoted by cytokines is NF-κB dependent in EoL-1 cells and human eosinophils. Moreover, it has been reported that MAPKs including ERK1/2, p38, and JNK are involved in ICAM-1 expression in human eosinophils and pulmonary epithelial cells (5, 15). In this study, we found that pretreatment of EoL-1 cells with JNK inhibitor SP600125, but not p38 inhibitor SB203580 or MEK1/2 inhibitor U0126, resulted in significant reduction of ICAM-1 expression stimulated by HDM extract. This suggests that only JNK MAPK is associated with ICAM-1 expression by HDM extract. Taken together, our results suggest that D. farinae extract induces ICAM-1 expression on EoL-1 cells via signaling pathways of NF-κB and JNK MAPK.
ICAM-1 expression on eosinophils is involved in degranulation and superoxide anion production (17, 18). A local increase of eosinophils expressing ICAM-1 has been found in inflammatory respiratory diseases such as asthma and idiopathic eosinophilic pneumonia (19, 20). Eosinophils in the sputum, but not blood, of symptomatic asthmatics not receiving steroid therapy were found to express ICAM-1 (21). Therefore, our findings suggest that D. farinae-induced ICAM-1 expression might contribute to amplification of eosinophil-mediated tissue inflammation in bronchial asthma.
Evidence indicates that several mite allergens are proteolytic enzymes (serine and cysteine proteases) and that the protease activity of the mite allergens sensitizes immune cells. For example, D. pteronyssinus allergen Der p 1 can induce upregulation of surface expression of ICAM-1 (CD54) on endothelial cells (22) and eosinophils (16). In addition, Der p 1 stimulates cytokine expression in airway epithelial cells through a protease-activated receptor-2 (PAR-2)-dependent mechanism (23). In contrast, human eosinophils are activated by cysteine protease allergen Der f 1 in a PAR-2 driven manner (24). Further studies on this issue will be required to unravel the immunopathological mechanisms of D. farinae-mediated allergic inflammation in bronchial asthma.
In summary, we report that D. farinae extract induces upregulation of the cell surface adhesion molecule ICAM-1 in EoL-1 cells through JNK and NF-κB pathways. Our findings may have clinical implications because the exposure to HDM antigens plays an important role in the regulation of eosinophilic inflammation in patients with bronchial asthma.
Figures and Tables
 | Fig. 1Effect of TNF-α and HDM extract on the expression of cell surface ICAM-1 on EoL-1 cells. EoL-1 cells were incubated with or without HDM extract (5-200 µg/mL) for 4.5 hr. TNF-α (20 ng/mL) was used as a positive control. Data are expressed as the mean±SEM of triplicate experiments. *, p<0.05 compared to cells incubated with medium alone. |
References
1. Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990. 323:1033–1039.

2. Jagels MA, Daffern PJ, Zuraw BL, Hugli TE. Mechanisms and regulation of polymorphonuclear leukocyte and eosinophil adherence to human airway epithelial cells. Am J Respir Cell Mol Biol. 1999. 21:418–427.

3. Tang ML, Fiscus LC. Important roles for L-selectin and ICAM-1 in the development of allergic airway inflammation in asthma. Pulm Pharmacol Ther. 2001. 14:203–210.
5. Wong CK, Ip WK, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor-induced adhesion molecule expression on eosinophils by p38 mitogen-activated protein kinase and nuclear factor-κB. Am J Respir Cell Mol Biol. 2003. 29:133–147.

6. Cheung PF, Wong CK, Ip WK, Lam CW. IL-25 regulates the expression of adhesion molecules on eosinophils: mechanism of eosinophilia in allergic inflammation. Allergy. 2006. 61:878–885.

7. Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997. 100:S2–S24.
8. Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995. 12:369–378.

9. Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998. 187:271–275.
10. Coward WR, Sagara H, Wilson SJ, Holgate ST, Church MK. Allergen activates peripheral blood eosinophil nuclear factor-κB to generate granulocyte macrophage-colony stimulating factor, tumor necrosis factor-α and interleukin-8. Clin Exp Allergy. 2004. 34:1071–1078.
12. Ip WK, Wong CK, Lam CW. Tumour necrosis factor-alpha-induced expression of intercellular adhesion molecule-1 on human eosinophilic leukaemia EoL-1 cells is mediated by the activation of nuclear factor-kappaB pathway. Clin Exp Allergy. 2003. 33:241–248.
13. Ree HI, Jeon SH, Lee IY, Hong CS, Lee DK. Fauna and geographical distribution of house dust mites in Korea. Korean J Parasitol. 1997. 35:9–17.

14. Holden NS, Catley MC, Cambridge LM, Barnes PJ, Newton R. ICAM-1 expression is highly NF-kappaB-dependent in A549 cells. No role for ERK and p38 MAPK. Eur J Biochem. 2004. 271:785–791.
15. Lin FS, Lin CC, Chien CS, Luo SF, Yang CM. Involvement of p42/p44 MAPK, JNK, and NF-κB in IL-1 β-induced ICAM-1 expression in human pulmonary epithelial cells. J Cell Physiol. 2005. 202:464–473.
16. Wong CK, Li ML, Wang CB, Ip WK, Tian YP, Lam CW. House dust mite allergen Der p 1 elevates the release of inflammatory cytokines and expression of adhesion molecules in co-culture of human eosinophils and bronchial epithelial cells. Int Immunol. 2006. 18:1327–1335.

17. Horie S, Okubo Y, Hossain M, Momose T, Suzuki J, Isobe M, Sekiguchi M. Intercellular adhesion molecule-1 on eosinophils is involved in eosinophil protein X release induced by cytokines. Immunology. 1997. 90:301–307.

18. Takashi S, Okubo Y, Horie S. Contribution of CD54 to human eosinophil and neutrophil superoxide production. J Appl Physiol. 2001. 91:613–622.

19. Mengelers HJ, Maikoe T, Brinkman L, Hooibrink B, Lammers JW, Koenderman L. Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994. 149:345–351.

20. Azuma M, Nakamura Y, Sano T, Okano Y, Sone S. Adhesion molecule expression on eosinophils in idiopathic eosinophilic pneumonia. Eur Respir J. 1996. 9:2494–2500.

21. Hansel TT, Braunstein JB, Walker C, Blaser K, Bruijnzeel PL, Virchow JC Jr, Virchow C Sr. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991. 86:271–277.

22. Mastrandrea F, Nicotra MR, De Vita L, Coradduzza G, Minardi A, Scarcia G, Manelli M, Cadario G, Parmiani S, Natali PG. Mite antigens enhance ICAM-1 and induce VCAM-1 expression on human umbilical vein endothelium. Allergol Immunopathol (Madr). 2003. 31:259–264.

23. Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002. 169:4572–4578.

24. Miike S, Kita H. Human eosinophils are activated by cysteine protease and release inflammatory mediators. J Allergy Clin Immunol. 2003. 111:704–713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download