Abstract
The purpose of this study was to demonstrate survival rate changes after the introduction of inhaled nitric oxide (iNO) therapy, and to identify the factors that influence these changes in neonates with a congenital diaphragmatic hernia (CDH) at a single center. A total of 48 neonates were divided into two groups based on the time of admission, i.e., into period I (P1; n=17; before the introduction of iNO therapy) and period II (P2; n=31; after the introduction of iNO therapy). Survival rates of the 48 neonates showed a tendency to increase from 53% during P1 to 77% during P2, but without a statistical significance, but a significant difference was found between survival rates during the two periods after adjusting for initial clinical characteristics, when the postoperative survival rate increased significantly from 69% for P1 to 100% for P2. The mean duration of preoperative respiratory management was significantly longer for P2 than for P1. Seven of 12 patients who received preoperative iNO therapy due to persistent pulmonary hypertension or refractory preductal hypoxemia in P2 survived after operation. We speculate that a management strategy based on iNO therapy and delayed operation, rather than differences between the initial clinical characteristics of the two study groups, might partially contribute to the observed improvements in postoperative and overall survival rates in neonates with CDH.
Congenital diaphragmatic hernia (CDH) occurs in 1 per 2,000-5,000 live births and affects approximately 1,100 infants annually in the U.S.A. (1). CDH remains one of the most challenging and perplexing malformations in neonatal intensive care units (NICUs), and shows a high mortality rate due to pulmonary hypoplasia and/or intractable persistent pulmonary hypertension (PPH), despite aggressive perinatal treatment. However, the relative rarity and clinical variability of CDH makes it difficult to conduct well-designed clinical studies at a single institution and to establish the most suitable treatment. Despite advances in neonatal intensive care over the last 2 decades, the overall survival rate of neonates with CDH (64%) has changed only slightly in recent years, and to date, uniform standards for CDH management have not been established (2-4).
Advanced therapies for CDH have included delayed operation, extracorporeal membrane oxygenation (ECMO), high-frequency oscillatory ventilation (HFOV), surfactant, and inhaled nitric oxide (iNO) therapy. However, the effects of these modalities in terms of statistically significant survival advantage in neonates with CDH have not been clearly determined. In particular, the effect of iNO on the outcome in CDH remains controversial (5-7).
In June 1998, iNO therapy was introduced as a new treatment modality in the NICU at Seoul National University Children's Hospital. Following the introduction of this therapy, we found that the survival rate of neonates with CDH had changed, and thus, we conducted this retrospective study to determine whether iNO therapy had influenced the survival rate, or whether the initial clinical characteristics of patients had co-incidentally become less severe. Thus, the purpose of this study was to determine survival rate changes after the introduction of iNO therapy, and to identify the factors that influence these changes in neonates with CDH at our institution.
The medical records of 55 CDH patients, admitted to Seoul National University Children's Hospital between 1 January 1990 and 31 May 2005, were reviewed retrospectively. Four infants who were admitted after the neonatal period, two neonates with chromosomal abnormalities or multiple malformations, and one neonate who succumbed to septic shock were excluded. A total of 48 neonates were included in this study and were divided into two groups according to the time point of admission, i.e., prior to or after the introduction of iNO therapy. Seventeen neonates were admitted during the period I (P1; January 1990 to May 1998; before the introduction of iNO therapy), and 31 neonates during the period II (P2; June 1998 to May 2005; after the introduction of iNO therapy). Data on patients' clinical characteristics and outcomes were collected and analyzed retrospectively. A flow diagram showing the course and outcome of all CDH patients enrolled in this study is presented in Fig. 1. The study was approved by the institutional research ethics committee.
To treat neonates with CDH showing respiratory distress, a conventional ventilator (CV) was generally started as the primary ventilation mode for inborn patients or on admission for outborn patients as follows; mean airway pressure (MAP), 5-10 mmHg; fraction of inspired O2 (FiO2), 0.4-1.0; and frequency, 40-60/min. If preoperative stabilization was not achieved using a CV (pH <7.25, PaCO2 >65 mmHg, and PaO2 <50 mmHg at MAP ≥10 mmHg, frequency ≥60/min, and FiO2 ≥0.6), HFOV was applied. Initial HFOV settings were as follows: FiO2, 0.6-1.0; frequency, 15 Hz; stroke volume, 10-15 mL; MAP, 2-3 cmH2O above the CV setting. In some patients, depending on the clinical state, HFOV was started as the primary ventilation mode. At our institution, an iNO delivery system (Metran Massmixer, Metran Co., Kawaguchi, Japan) and a high-frequency oscillatory ventilator (Humming V, Metran Co., Kawaguchi, Japan) were first introduced in June 1998 and in August 1996, respectively. During P2, iNO was administered in addition to HFOV when PPH (right-to-left shunt on echocardiogram, difference between preductal and postductal saturation of oxygen >10%) or refractory preductal hypoxemia (pH <7.25, PaO2 <50 mmHg, and SaO2 <85% at MAP ≥15 mmHg and FiO2 ≥0.6) developed despite HFOV. The concentration of iNO was initiated at 5-20 ppm and increased to a maximum of 80 ppm. Reduction of the oxygenation index (OI=[MAP×FiO2×100]/PaO2) to 30% or more within 24 hr was defined as a clinical response, and a reduction of OI to under 30% or increase of OI within 24 hr was defined as a clinical no-response (8). Respiratory management data were collected and analyzed. During P1 and P2, a CDH repair operation was performed when clinical conditions had been stabilized to some degree (pH ≥7.25, OI <12, and stable vital signs). The operation was not performed when clinical conditions had not stabilized (pH <7.25, OI ≥12, and unstable vital signs). Postoperative respiratory management was performed as described for the preoperative protocol.
Data were presented as means±standard deviations. Continuous variables were compared using the Mann-Whitney U test. Categorical variables were compared using the chi-square and Fishers exact tests. p values of less than 0.05 were considered significant. Survival analysis was based on 7 days of survival data. Survival curves were constructed using the Kaplan-Meier method. Cox regression analysis was used to determine whether the survival rates of the periods were different and independent of initial clinical characteristics. Statistical analysis was performed using SPSS version 12.0.
Clinical characteristics of the patients enrolled during the two periods are given in Table 1. The rates of inborn delivery and prenatal diagnosis were significantly higher during P2 than during P1. No significant differences were observed between the two periods in terms of any other clinical characteristics.
The overall survival rate of the 48 CDH patients was 69% (33 of 48), and the survival rate increased from 53% (9 of 17) during P1 to 77% (24 of 31) during P2 (Table 1). All deaths (15 patients) occurred before six days of life (2-146 hr), and eight patients died before 24 hr of life. All deaths occurred as a result of PPH or refractory hypoxemia. When survival rates over the first 7 days after birth were analyzed using Kaplan-Meier curves for the two periods (not shown), the survival rate for P2 was higher than that for P1, but without a significant difference (p=0.096). However, after adjusting for the initial clinical characteristics (sex, gestational age, birth weight, Apgar score at 5 min, significant associated anomalies, prenatal diagnosis, inborn delivery, polyhydramnios, and liver herniation), this difference between the two periods achieved a significance (p=0.033) (Table 2). Thirty-seven of the 48 patients (P1, 13 of 17; P2, 24 of 31) underwent an operation (Table 3). Postoperative survival rates increased significantly from 69% (9 of 13) in P1 to 100% (24 of 24) in P2.
Pre- and postoperative respiratory managements during the two periods are summarized in Table 3. The number of patients who received HFOV therapy before operation was not significantly different between the two periods. However, the mean durations of preoperative intubation and mechanical ventilation were significantly longer in P2 than in P1, and the mean duration of preoperative mechanical ventilation in survivors was also significantly longer in P2 than in P1 (data not shown, 13.1±7.9 hr vs. 40.6±24.3 hr, p=0.003). Mean maximal preoperative peak inspiratory pressure (PIP), MAP, and FiO2 were not significantly different in P1 and P2. Mean maximal preoperative PIP, MAP, and FiO2 in P2 were measured before iNO therapy.
Twelve of 31 patients in P2 received iNO therapy (Fig. 2). Initial OI was above 12 in all patients who received iNO therapy (data not shown). Seven of the 12 were regarded as clinical responders and five as non-responders. Six of the seven patients in the clinical response group underwent operation, and five of the six patients survived after postoperative iNO therapy, and one patient survived without postoperative iNO. Only one of the five patients in the clinical no-response group underwent operation, and survived after postoperative iNO therapy. This patient had the lowest initial OI (=12) in the clinical no-response group. In total, 7 of 12 patients who received iNO therapy in P2 survived postoperatively.
Comparisons of survival rates of CDH patients between institutions or periods are difficult and controversial because the spectrum of disease severity is very wide. In recent retrospective reviews of CDH cases in the United Kingdom (a 13-yr study) and France (a 5-yr study), overall survival rates ranging between 50% and 56% were observed (9, 10). More recently, the Congenital Diaphragmatic Hernia Study Group reported an overall survival rate of 64% based on data from 71 institutions in North America, Europe, and Australia (4). Thus, the overall survival rate of 69% at our institution (a 15-yr study) is similar to or higher than those reported from other institutions (2, 4, 9-11). However, the survival rate of 69% at our institution has not been constant over the 15-yr period. After iNO therapy was introduced in June 1998, we noticed a change in the survival rates of neonates with CDH. Thus, to investigate this change in survival rate, we divided the study cohort into two groups according to the admission date, i.e., prior to (P1) or after (P2) the introduction of iNO therapy. Increases in inborn delivery and prenatal diagnosis in P2 can be explained by the fact that prenatal diagnosis has gradually become routine with the advent of frequent screening ultrasonography during pregnancy, and thus, more mothers with a prenatal diagnosis of CDH are referred to a tertiary-care center equipped with 'immediate planned care'.
No significant difference was observed between the survival rates of the two periods by Kaplan-Meier curve analysis, despite the fact that the survival rate obtained after the introduction of iNO therapy was higher than during the previous period. Thus, additional evaluation was necessary because ranges of severity and clinical variability in CDH at the time of birth made it difficult to simply compare the survival rates of the two periods. Therefore, we compared period survival rates after adjusting for initial clinical characteristics related to the survival rate, i.e., sex, gestational age, birth weight (4), Apgar score at 5 min (4), significant associated anomalies (7, 12-14), prenatal diagnosis, inborn delivery (12, 15), polyhydramnios (16), and liver herniation (17). Among the above initial clinical characteristics, birth weight and Apgar score at 5 min were found to be the most important predictors to estimate the severity of CDH in the first 5 min of life by the Congenital Diaphragmatic Hernia Study Group (4). The lung-to-head ratio (18), one of the reliable predictors to help determine postnatal survival in CDH, was excluded from survival analysis because the rate of outborn delivery without prenatal diagnosis was very high at our institution. However, we considered that the significance of the lung-to-head ratio could be reflected to some degree by initial clinical characteristics such as Apgar score at 5 min. We also excluded clinical characteristics such as pneumothorax caused by CDH management from survival analysis. According to this analysis, the survival rate of P2 was significantly higher than that of P1. This result suggests that the survival rate difference observed between the two periods is likely to have been influenced by the change of management strategy rather than due to differences between the above initial clinical characteristics, including prenatal diagnosis and inborn delivery, which were significantly different between P1 and P2.
The significant differences in the management strategy between the two periods in this study were the introduction of iNO therapy and the longer duration of preoperative respiratory management in P2. Preoperative HFOV therapy was excluded from evaluation of further management strategy because the number of patients who received HFOV therapy preoperatively was not significantly different between the two periods. The most important aspect of the postnatal management of neonates with CDH is PPH management (7). NO, originally identified as endothelial derived relaxing factor, has been used successfully to treat PPH because it is a potent vasodilator (19). Some investigators have tried iNO therapy in neonates with PPH complicating the course of CDH (20, 21). However, a multicenter randomized controlled trial of iNO therapy in CDH patients failed to demonstrate any reduction in the need for ECMO or in mortality (5). These results are consistent with previous observations by another group, who found that early iNO therapy was not associated with significant improvements in oxygenation in neonates with CDH (22). On the other hand, other studies favor the effect of iNO therapy in neonates with CDH, although these were not controlled studies (15, 23). Recently, Downard et al. suggested that iNO therapy could be an important adjunct for treating PPH in neonates with CDH, when used in concert with echo-cardiography (7). The results of our study seem to support those of previous studies, which found that iNO therapy is effective in the management of CDH. In the present study, the postoperative survival rate in the P2 group was significantly higher. Among the 12 patients who received iNO therapy as a rescue therapy for PPH and refractory hypoxemia in P2, seven patients survived postoperatively. It is possible that the survival of these seven patients in P2 was partly attributable to the introduction of iNO therapy. We mentioned above that a reduction in the oxygenation index to 30% or more within 24 hr of iNO therapy was defined as a clinical response (8). This definition is meaningful in that most of the clinical responders (6 of 7) in P2 survived after operation, and thus contributed to improved survival in P2. Another significant difference in management strategies in this study was the longer duration of preoperative respiratory management in P2. Several researchers have suggested that delayed operation for CDH after prolonged stabilization for at least 24 hr has a beneficial effect on survival rate for patients with CDH (2, 7, 24). In our study, we believe that iNO therapy is one of the factors that made delayed operation possible in P2, although more severe preoperative respiratory conditions may have been responsible for these delays.
Some recent studies similar to our own allow comparisons of the survival rates of neonates with CDH with historical controls (23, 25-27). Three of these studies reported dramatically improved survival rates in neonates with CDH during 1988-2000, 1978-2001, and 1996-2001, respectively (23, 26, 27). However, only Okazaki et al. reported that the survival rate of neonates with CDH was improved significantly after the introduction of iNO therapy with a delayed operation (26). One of the most important limitations of the above studies, including the present study, concerns the use of a historical control, which may mislead investigators believe that changes in survival rate over a period is the direct result of a new therapy. All of the above studies did not prove statistically that the improved survival rate observed in the later period was not related to a less severe clinical condition. As mentioned above, the spectrum of severity of CDH is very wide, and the determination of effects of some specified treatment for this disease is very difficult. In this study, we tried to adjust for some initial clinical characteristics that might have influenced survival rates mainly, and to minimize confounding features of a study using a historical control. However, present study also has some limitations to adjust for all initial clinical characteristics due to its retrospective nature as other previous studies. Further prospective studies will be required to overcome these limitations and to confirm our hypothesis raised in this study.
In summary, a significant improvement in the survival rate of neonates with CDH after the introduction of iNO therapy was observed after adjusting for initial clinical characteristics. The improved survival of neonates with CDH seems to be associated with an improved postoperative survival rate after the introduction of iNO therapy. We speculate that a management strategy based on iNO therapy and delayed operation, rather than differences between the initial clinical characteristics of the two study groups, might have partially contributed to the improvements observed in postoperative and overall survival rates in neonates with CDH.
Figures and Tables
Fig. 1
Flow diagram showing the courses and outcomes of the 55 patients with congenital diaphragmatic hernia (CDH) enrolled in this study. NICU, neonatal intensive care unit.

Fig. 2
Outcomes of pre- and postoperative iNO therapy for neonates with congenital diaphragmatic hernia during period II.
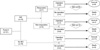
Table 2
Changes in survival rates between periods I and II after adjusting for the initial clinical characteristics* (by Cox regression analysis)

References
1. Weinstein S, Stolar CJ. Newborn surgical emergency, congenital diaphragmatic hernia and extracorporeal membrane oxygenation. Pediatr Clin North Am. 1993. 40:1315–1333.
2. Clark RH, Hardin WD Jr, Hirschl RB, Jaksic T, Lally KP, Langham MR Jr, Wilson JM. Current surgical management of congenital diaphragmatic hernia: a report from the congenital diaphragmatic hernia study group. J Pediatr Surg. 1998. 33:1004–1009.

3. Beresford MW, Shaw NJ. Outcome of congenital diaphragmatic hernia. Pediatr Pulmonol. 2000. 30:249–256.

4. The Congenital Diaphragmatic Hernia Study Group. Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001. 36:141–145.
5. The Neonatal Inhaled Nitric Oxide Study Group (NINOSG). NINOSG). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics. 1997. 99:838–845.
7. Downard CD, Wilson JM. Current therapy of infants with congenital diaphragmatic hernia. Semin Neonatol. 2003. 8:215–221.

8. Dimitriou G, Greenough A, Kavvadia V, Devane SP, Rennie JM. Outcome predictors in nitric oxide treated preterm infants. Eur J Pediatr. 1999. 158:589–591.

9. Dillon E, Renwick M, Wright C. Congenital diaphragmatic hernia: antenatal detection and outcome. Br J Radiol. 2000. 73:360–365.
10. Dubois A, Storme L, Jaillard S, Truffert P, Riou Y, Rakza T, Pierrat V, Gottrand F, Pruvot FR, Leclerc F, Lequien P. Congenital hernia of the diaphragm. A retrospective study of 123 cases recorded in the Neonatal Medicine Department, URHC in Lille between 1985 and 1996. Arch Pediatr. 2000. 7:132–142.
11. Reickert CA, Hirschl RB, Atkinson JB, Dudell G, Georgeson K, Glick P, Greenspan J, Kays D, Klein M, Lally KP, Mahaffey S, Ryckman F, Sawin R, Short BL, Stolar CJ, Thompson A, Wilson JM. Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery. 1998. 123:305–310.

12. Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: a meta-analysis of mortality factors. J Pediatr Surg. 2000. 35:1187–1197.

13. Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002. 37:357–366.

14. Bedoyan JK, Blackwell SC, Treadwell MC, Johnson A, Klein MD. Congenital diaphragmatic hernia: associated anomalies and antenatal diagnosis. Pediatr Surg Int. 2004. 20:170–176.

15. Bétrémieux P, Gaillot T, de la Pintiere A, Beuchee A, Pasquier L, Habonimana E, Le Bouar G, Branger B, Milon J, Fremond B, Wodey E, Odent S, Poulain P, Pladys P. Congenital diaphragmatic hernia: prenatal diagnosis permits immediate intensive care with high survival rate in isolated cases. A population-based study. Prenat Diagn. 2004. 24:487–493.

16. Adzick NS, Vacanti JP, Lillehei CW, O'Rourke PP, Crone RK, Wilson JM. Fetal diaphragmatic hernia: ultrasound and clinical outcome in 38 cases. J Pediatr Surg. 1989. 24:654–658.
17. Albanese CT, Lopoo J, Goldstein RB, Filly RA, Feldstein VA, Calen PW, Jennings RW, Farrell JA, Harrison MR. Fetal liver position and perinatal outcome for congenital diaphragmatic hernia. Prenat Diagn. 1998. 18:1138–1142.

18. Laudy JA, Van Gucht M, Van Dooren MF, Wladimiroff JW, Tibboel D. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diagn. 2003. 23:634–639.

19. Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, deLemos RA, Sardesai S, McCurnin DC, Moreland SG, Cutter GR, Abman SH. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997. 131:55–62.

20. Bohn DJ, Pearl R, Irish MS, Glick PL. Postnatal management of congenital diaphragmatic hernia. Clin Perinatol. 1996. 23:843–872.

21. Henneberg SW, Jepsen S, Andersen PK, Pedersen SA. Inhalation of nitric oxide as a treatment of pulmonary hypertension in congenital diaphragmatic hernia. J Pediatr Surg. 1995. 30:853–855.

22. Karamanoukian HL, Glick PL, Zayek M, Steinhorn RH, Zwass MS, Fineman JR, Morin FC 3rd. Inhaled nitric oxide in congenital hypoplasia of the lungs due to diaphragmatic hernia or oligohydramnios. Pediatrics. 1994. 94:715–718.
23. Okuyama H, Kubota A, Oue T, Kuroda S, Ikegami R, Kamiyama M, Kitayama Y, Yagi M. Inhaled nitric oxide with early surgery improves the outcome of antenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 2002. 37:1188–1190.

24. Reyes C, Chang LK, Waffarn F, Mir H, Warden MJ, Sills J. Delayed repair of congenital diaphragmatic hernia with early high-frequency oscillatory ventilation during preoperative stabilization. J Pediatr Surg. 1998. 33:1010–1014.
25. Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003. 112:532–535.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download