Abstract
The observation that human matrix metalloproteinase (MMP)-8 is over-expressed in ectatic bronchi in patients with bronchiectasis suggests that polymorphisms altering the expression of MMP-8 may contribute to the susceptibility to development of bronchiectasis. We evaluated the association between the presence of bronchiectasis in a Korean population and two single nucleotide polymorphisms (SNPs) (-799C/T and -381A/G) on the promoter region of the MMP-8 gene that are reported to alter the promoter activity and thereby the gene expression. Genotyping through polymerase chain reaction (PCR) and subsequent automatic sequencing was done in 167 patients with bronchiectasis and their age-, sex-matched healthy controls to reveal that only -799C/T is polymorphic among Koreans. In the patient group with bronchiectasis, the frequency of -799C/C, C/T, and T/T genotypes were 41.9%, 49.7%, and 8.4%, respectively. A similar distribution was observed in the control group: C/C (49.7%), C/T (43.1%), and T/T (7.2%) (p=0.36). In subgroup analysis, no significant difference was observed among the patients according to; the extent of disease (p=0.76), colonization of microorganisms (p=0.56), or association of mycobacteria (p=0.17). From these results, we conclude that -799C/T on the promoter region of MMP-8 lacks association with development of bronchiectasis in Koreans.
Bronchiectasis is a pathologic state of airway dilatation as a result of continuous airway inflammation and destruction, which forms a vicious circle with recurrent infection. Although bronchiectasis is an uncommon disease and little is known about its prevalence worldwide, the disease potentially cause devastating illnesses, including repeated infections requiring antibiotics, disabling productive cough, shortness of breath, and occasional hemoptysis (1). While the pathogenesis of bronchiectasis is not fully elucidated, it has been understood that the destruction of the bronchial wall caused by continued extracellular matrix (ECM) damage is a major contributor to the development of permanent airway dilatation (1, 2).
The matrix-metalloproteinases are a family of proteolytic enzymes that degrade the main protein components of the extracellular matrices (ECMs) and may lead to continued airway destruction when miscontrolled (3). Previous studies showed that an excessive MMP activity plays an important role in the pathogenesis of several respiratory diseases including asthma, chronic obstructive pulmonary disease (COPD), adult respiratory distress syndrome (ARDS), and pulmonary fibrosis (4-7). Amongst them, MMP-8, in particular, is reported to be overly expressed in the pulmonary tissue and lavage fluid from patients with bronchiectasis to suggest its potential role in the development of bronchiectasis (2, 8-11). In the meantime, two single nucleotide polymorphisms (-799C/T and -381A/G) were identified from the promoter region of the MMP-8 gene and reported to alter the promoter activity and subsequent gene expression (12).
In this context, the present study aimed primarily at examining the association between the SNPs of MMP-8 and the presence of bronchiectasis in a Korean population. Moreover, the frequencies of each genotype were reviewed in view of the correlation of the polymorphism and the extent of disease, presence of microorganism colonization, and the degree of pulmonary functional impairment, among the patient group.
Since the prevalence of SNP on -799C of MMP-8 promoter is unknown in Korean population, estimation was made according to the known value for a Western population, 70% (12). Determination factors thereby included: an assumed prevalence of 70%, alpha error of 0.10, beta error of 0.20, and the expected difference being 15%. Substitution of these parameters to the sample size table by Manchin et al. has yielded the minimum sample size of 128 (13).
We prospectively enrolled 167 patients with bronchiectasis diagnosed based on clinical manifestation and radiographic findings on high-resolution computerized tomography (HRCT) or conventional computerized tomography of the chest. The definition of 'ectasis' is as enlarged internal bronchial diameter, where the bronchi appear larger than the accompanying artery. Patients with definite predisposing factors of bronchiectasis such as primary ciliary dyskinesia, and various immune-deficient states were excluded from the study. In addition, patients with diffuse panbronchiolitis or allergic bronchopulmonary aspergillosis were also excluded. As the control group, we enrolled 167 age- and sex-matched healthy blood donors. All the patient and control participants were recruited from Seoul National University Hospital, and the data had been used for another previous study (14). The protocol of this study was approved by the Institutional Review Board of Seoul National University Hospital, and informed consent was obtained before participation from all subjects.
Each blood sample drawn into EDTA tubes went through a DNA extraction process using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN, U.S.A.) according to the manufacturer's protocol. On the basis of the human MMP-8 promoter sequence deposited in GenBank (accession no. AF059679), a promoter fragment was amplified with a forward primer sequence of 5'-CTGTTGAAGGCCTAGAGCTGCTGCTCC-3' (corresponding to bp -872 to -846) and a reverse primer 5'-CATCTTCTCTTCAAACTCTACCC-3' (corresponding to bp +74 to +96) yielding a 968-bp product in accordance with the previous report (12). The PCR was performed in a 50 µL reaction volume containing 100 ng genomic DNA, 0.5 pM of each primer, 0.2 mM dNTPs, 10x reaction buffer, 1.5 mM MgCl2 and 2.5 units of Taq-polymerase (Invitrogen, Carlsbad, CA, U.S.A.) in the 9600 Gene Amp PCR Thermal Cycler. After purifying PCR products with a commercialized kit (QIAGEN, Valencia, CA, U.S.A.), they were sequenced using an ABI 377 sequencer (Applied Biosystems, Foster City, CA, U.S.A.) according to the manufacturer's protocol.
Association between SNP on -799C/T and bronchiectasis was tested using the two-tailed chi-square test, where the p value below 0.05 being considered significant. Distribution of the alleles and genotypes were examined to determine differences among the patient and control groups and also in the following aspects: according to the extent of bronchiectasis, the presence of microorganism colonization, and the association of mycobacteria. Odds ratios and 95% confidence intervals were calculated to assess the relative risks conferred. SPSS version 12.0 (SPSS Inc, Chicago, IL, U.S.A.) was used for all statistical analyses.
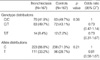
A total of 167 patients with bronchiectasis (77 [46.1%] females, median age 58 [23-80] yr) were recruited. Fifty-three of them (37.1%) were current or ex-smokers, and 162 had history of respiratory disease including tuberculosis (62 patients), measles (39 patients), pertussis (18 patients), and sinusitis (19%). One hundred and nineteen of the patients were collected for sputum samples, and various micro-organism were cultured in 45 of them (37%, of 119): Streptococcus (26 patients), nontuberculous mycobacteria (24 patients), and Pseudomonas aeruginosa (12 patients). As for the radiological extent of disease, more than two lobes were involved in 58% (97/167) of the patients. The mean percentage of predicted FEV1 (forced expiratory volume in one second) and FVC (forced vital capacity) were 80.1±24.0% and 91.3±19.0%, respectively (Table 1).
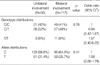
In an intention to detect other possible SNPs in Koreans, we additionally screened every sequenced result. However, no additional SNP was identified except -799C/T. In patients with bronchiectasis, the frequency of -799C/C, C/T, and T/T genotypes were 41.9%, 49.7%, and 8.4%, respectively. A similar distribution was observed in the control group: C/C (49.7%), C/T (43.1%), and T/T (7.2%). Although the distribution of genotypes were in Hardy-Weinberg equilibrium, the difference of genotype distribution as well as that of allele's was not statistically significant (p=0.36 and 0.21) (Table 2).
In subgroup analysis, no significant difference in terms of genotypes or alleles was observed among the patients according to: the extent of disease (p=0.76 for genotypes, 0.75 for alleles), colonization of microorganisms (p=0.56 and 0.65) or the association of mycobacteria (p=0.17 and 0.13) (Tables 3, T4, 5).
This study showed no significant association between SNPs on MMP-8 promoter gene and bronchiectasis in Koreans. We studied two previously reported SNPs, and one of them (-799C/T) was shown polymorphic among the population. We searched for additional polymorphisms, only to observe the presence of the previously shown one (-799C/T). No significant difference was observed in either allelic or genotypic distribution of -799C/T between the patients, and controls, and this SNP showed no correlation with the presence of bronchiectasis, disease extent, or mycobacterial diseases/infection as a cause of bronchiectasis in the study population.
Assumable from being so-called the 'orphan disease,' little is known about the pathogenesis of bronchiectasis despite considerable recent progress made in the understandings in other respiratory diseases. Several localized and generalized impairments of host defense mechanisms have been proposed as the causes for developing bronchiectasis, such as primary ciliary dyskinesia, congenital immunodeficiency, and cystic fibrosis (15, 16). However, the rarity of the above-mentioned syndromes in Koreans suggests that other genetic factors may contribute to the risk of bronchiectasis (17-19).
The matrix metalloproteinase family, ever since its initial identification in the involuting tail of tadpoles by their ability to degrade collagen, has been studied for its physiological roles and pathological contribution to tissue-remodeling diseases. While several studies have implicated an excess MMP activity in the pathogenesis of destructive pulmonary pathology including COPD, asthma, and ARDS (4-7, 20), MMP-8 in particular is reported to harbor stronger correlation with one of the most destructive pathology, bronchiectasis (2, 9). Meanwhile, there have been efforts to analyze the MMPs' association with obstetrical diseases where extracellular matrix (ECM) remodeling is an important process in several phases of human parturition. As a result, a specific genetic correlation between MMP-8 and preterm premature rupture of membranes was reported; the presence of SNPs and their functional significance upon the gene expression (12).
This study, inspired by the previous studies, had proposed a role of the previously identified SNPs (12) on the pathogenesis of bronchiectasis. Although the present study could not reveal a significant correlation with those specific polymorphisms, MMPs still deserve attention, considering its proposed central role in ECM turnover and the development of several destructive pathologies. Alternative efforts to investigate on additional possible genetic association between MMPs and bronchiectasis are needed.
Figures and Tables
References
2. Zheng L, Lam WK, Tipoe GL, Shum IH, Yan C, Leung R, Sun J, Ooi GC, Tsang KW. Overexpression of matrix metalloproteinase-8 and -9 in bronchiectatic airways in vivo. Eur Respir J. 2002. 20:170–176.

3. Woessner JF. Parks W. C., Mecham R. P., editors. Matrix Metalloproteinase. The matrix metalloproteinase family. 1998. San Diego: Academic Press;1–44.
4. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998. 102:783–788.

5. Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000. 117:684–694.

6. Shapiro SD, Senior RM. Matrix metalloproteinases. Matrix degradation and more. Am J Respir Cell Mol Biol. 1999. 20:1100–1102.
7. Vignola AM, Riccobono L, Mirabella A, Profita M, Chanez P, Bellia V, Mautino G, D'Accardi P, Bousquet J, Bonsignore G. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998. 158:1945–1950.

8. Prikk K, Maisi P, Pirila E, Sepper R, Salo T, Wahlgren J, Sorsa T. In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. J Pathol. 2001. 194:232–238.

9. Sepper R, Konttinen YT, Ding Y, Takagi M, Sorsa T. Human neutrophil collagenase (MMP-8), identified in bronchiectasis BAL fluid, correlates with severity of disease. Chest. 1995. 107:1641–1647.

10. Sepper R, Konttinen YT, Buo L, Eklund KK, Lauhio A, Sorsa T, Tschesche H, Aasen AO, Sillastu H. Potentiative effects of neutral proteinases in an inflamed lung: relationship of neutrophil procollagenase (proMMP-8) to plasmin, cathepsin G and tryptase in bronchiectasis in vivo. Eur Respir J. 1997. 10:2788–2793.
11. Tsang KW, Chan K, Ho P, Zheng L, Ooi GC, Ho JC, Lam W. Sputum elastase in steady-state bronchiectasis. Chest. 2000. 117:420–426.

12. Wang H, Parry S, Macones G, Sammel MD, Ferrand PE, Kuivaniemi H, Tromp G, Halder I, Shriver MD, Romero R, Strauss JF 3rd. Functionally significant SNP MMP8 promoter haplotypes and preterm premature rupture of membranes (PPROM). Hum Mol Genet. 2004. 13:2659–2669.

13. Manchin D, Campbell MJ, Fayers PM, Pinol AP. Sample Size Tables for Clinical Studies. 1997. 2ed. Blackwell Sience.
14. Kim HJ, Lee HW, Lee JE, Joh JS, Han SK, Shim YS, Yim JJ. Lack of association between the microsatellite polymorphism in intron 2 of human toll-like receptor 2 gene and bronchiectasis among Koreans. Respirology. 2007. 12:49–53.

15. Stead A, Douglas JG, Broadfoot CJ, Kaminski ER, Herriot R. Humoral immunity and bronchiectasis. Clin Exp Immunol. 2002. 130:325–330.

16. Verra F, Escudier E, Bignon J, Pinchon MC, Boucherat M, Bernaudin JF, de Cremoux H. Inherited factors in diffuse bronchiectasis in the adult: a prospective study. Eur Respir J. 1991. 4:937–944.
17. Park JE, Chang YW, Lim SH, Lee JI, Kang HM, Choi YK. The immotile cilia syndrome. Korean J Int Med. 1984. 27:1508–1512.
18. Chi JG, Yoon CJ. Primary ciliary dyskinesia (Immotile Cilia Syndrome)-Clinical and electron microscopic analysis of 17 cases. Korean J Pathol. 1993. 27:99–107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download