Abstract
In this study, we compared the paramedian interfascial approach (PIA) and the traditional midline approach (MA) for lumbar fusion to determine which approach resulted in the least amount of postoperative back muscle atrophy. We performed unilateral transforaminal posterior lumbar interbody fusion via MA on the symptomatic side and pedicle screw fixation via PIA on the other side in the same patient. We evaluated the damage to the paraspinal muscle after MA and PIA by measuring the preoperative and postoperative paraspinal muscle volume in 26 patients. The preoperative and postoperative cross-sectional area, thickness, and width of the multifidus muscle were measured by computed tomography. The degree of postoperative paraspinal muscle atrophy was significantly greater on the MA side than on the contralateral PIA side (-20.7% and -4.8%, respectively, p<0.01). In conclusion, the PIA for lumbar fusion yielded successful outcomes for the preservation of paraspinal muscle in these 26 patients. We suggest that the success of PIA is due to less manipulation and retraction of the paraspinal muscle and further studies on this technique may help confirm whether less muscle injury has positive effects on the long-term clinical outcome.
A commonly chosen surgical treatment for symptomatic lumbar stenosis with instability is transforaminal lumbar interbody fusion (TLIF) with pedicle screw fixation (PSF). The procedure allows stabilization and support of the anterior column of the spine while allowing direct nerve root decompression from a posterior approach (1). Posterior as well as anterior column stability is achieved when PSF is added. Moreover, unilateral TLIF with PSF is favorable because it causes less muscle injury. However, postoperative low back pain is one of the major problems in the so-called failed back surgery syndrome (2). The deflection of muscle from the spinal processes, and subsequent prolonged wide retraction of conventional lumbar fusion, can result in ischemia and denervation of the paraspinal musculature, which may lead to postoperative muscle atrophy and pain (2-5). The multifidus muscle is the most vulnerable to injury during posterior spinal surgery, as it is innervated only by the medial branch of the dorsal ramus, with no intersegmental nerve supply as in the other paraspinal muscles (6). The medial branch of the dorsal rami is very vulnerable to compression during lateral displacement of muscle mass at surgery, particularly where the nerve is relatively fixed as it runs under the fibro-osseous mamilloaccessory ligament (2, 7).
Therefore, there has been increased enthusiasm for the development of minimally invasive operating techniques that spare the dorsal rami and also minimize approach-related morbidities, while achieving the same results as the more traditional invasive approaches (8). We suggest that minimally invasive muscle sparing approaches to posterior lumbar fusion procedures can be accomplished through the paramedian interfascial approach (PIA) technique, thus minimizing muscle disruption and ischemic damage. The PIA allows manual dissection between the multifidus and longissimus muscles and easy exposure to the transverse process and lateral aspect of the facet joint with minimal retraction (9). We compared the two different approach techniques on the paraspinal muscles in the same patient. We suggest that this study design allows the evaluation of the postoperative changes in paraspinal muscles objectively and independently of individual clinical outcomes.
The purpose of our study was to determine whether the paramedian muscle-splitting approach for lumbar fusion resulted in less paraspinal muscle atrophy than the conventional midline approach (MA).
This study was conducted by retrospective case selection and observation of postoperative changes of the multifidus muscle volume after PIA and MA. We included 26 patients who underwent unilateral TLIF with ipsilateral PSF via MA and contralateral PSF via PIA for degenerative conditions of the lumbar spine, and who were resistant to conservative treatment from January 2005 to February 2006. They were enrolled in the pilot computed tomography (CT) study. Patients were excluded if they had 1) a history of a previous back operation, 2) atrophy of the paraspinal musculature on preoperative magnetic resonance imaging, 3) the inability to undergo follow-up CT at our institution, 4) a spinal malignancy, or 5) a spinal infection.
All of the surgeries were performed by a surgeon (one of the authors) at a single institution. The study group included 11 male patients and 15 female patients. The mean age at surgery was 52 yr (range, 26-69 yr), and the average follow-up period was 11 months (range, 6-18 months).
All patients underwent surgery in the prone position. The detailed surgical techniques of TLIF are described elsewhere (10). The side chosen for TLIF via MA was based on the location of the preoperative radicular symptoms.
A total facetectomy on the symptomatic side was performed using an osteotome and Kerrison rongeurs. The inferior facet joint was removed, after which the tip of the superior facet was excised. The resected bones were saved for use as interbody graft material. Then, the ligamentum flavum was resected laterally to visualize the ipsilateral exiting and traversing nerve roots. After conducting the discectomy, two cages filled with auto- and allograft bone material were inserted. If required, cancellous bone was harvested from the iliac spine via a 1- to 2-cm incision. Before the second cage insertion, previously harvested iliac crest bone graft mixed with local bone was tightly packed into the anterior disc space to facilitate bony fusion. The pedicle screw (PS) insertion was then performed on the ipsilateral side.
Although a separated paramedian skin incision had been used early in this study, some patients also received a midline skin incision followed by a paramedian fascial incision 1 to 2 cm lateral to the midline. Through the fascial incision, a muscle-splitting technique using finger dissection between multifidus and longissimus muscles was used to gain access to the contralateral facet joint. Access was established with blunt finger or Cobb periosteal dissection. Once the facets were palpated and the capsule was accessible, the contralateral transverse processes and pars interarticularis were exposed, as well as the caudal facet. The PS insertion was then performed on the contralateral side (Fig. 1). Our preferable entry points for PSF were at the intersection of the axial plane through the middle of the transverse processes and the sagittal plane through the superior facet. After the pedicle was sounded with a probe, the hole was tapped and inspected with a fine ball-tip probe, and a screw was then inserted. The fascia was closed with 1-0 Vicryl, and the subcutaneous tissues were apposed with 3-0 Vicryl sutures. Most patients were instructed to wear a lumbar corset when they were out of bed for the 1 to 1.5 months following surgery.
The cross-sectional area, thickness, and width of the multifidus muscle were measured on the CT slice parallel to the disc space corresponding to the operation, preoperatively and postoperatively (Fig. 2). Direct visualization of the multifidus muscle at the level of fusion was inadequate because of interference by the metal artifact of the screws and rods. A 5-mm-thick, axial image was made at the supra-adjacent disc space of the fused segment on follow-up CT, and the most inferior axial image without metal artifact was selected for evaluation. The average of the measurements from the two selected axial CT images was calculated. The measurements were obtained with an image analyzer program (OPTIMAS 6.5, Optimas, Inc., Bothell, WA, U.S.A.). The CT scans were performed approximately 6 months postoperatively (range 6-18 months) because muscle swelling continued as long as 6 months after surgery and then reduced as the edema subsided (11, 12). The cross-sectional area, thickness, and width of the multifidus muscle were measured on the contralateral PIA side as well as the ipsilateral MA side. These measurements were performed by one of the authors.
For the statistical analysis, SPSS software (version 12.0, 2003; SPSS, Inc., Chicago, IL, U.S.A.) was used. The paired t-test was used for statistical analysis of the differences in noncategorical variables between preoperative and postoperative assessments. A p value less than 0.05 was considered statistically significant.
The postoperative changes of the cross-sectional area of the multifidus muscle on the MA and PIA sides are shown in Fig. 4. The results showed that there was a significant decrease in the cross-sectional area of multifidus muscle on the side of the MA, with an area of 1,121.3±235.7 mm2 on the preoperative CT, compared to 889.4±241.9 mm2 on the follow-up CT (-20.7%, p=0.002). By contrast, the results on the side of PIA showed no statistical difference in the cross sectional area of the multifidus muscle between preoperative (1,122.9±246.0 mm2) and follow-up CT (1,069.5±252.1 mm2) (-4.8%, p>0.05).
The paraspinal muscle thickness did not change significantly in the early stage (less than 8 months) in most patients, although a few showed an increase on the PIA side. There was a visual difference in the late postoperative muscle thickness between the MA and PIA sides estimated from the axial CT images (Fig. 5). However, there was no statistically significant difference in the multifidus width on the MA and PIA sides between preoperative and follow-up (Table 1).
Paraspinal muscle thickness on the side of MA decreased postoperatively in all patients (39.8±5.6 mm preoperatively, 35.5±5.2 mm postoperatively). Paraspinal muscle thickness on the PIA side decreased slightly after the surgery in all but three patients (39.8±5.6 mm preoperatively, 39.3±5.7 mm postoperatively). The change in paraspinal muscle thickness at more than 8 months postoperatively was -10% in males and -13.5% in females operated on via MA, and -0.3% in males and -4.4% in females operated on via PIA. The decrease was significantly larger on the side of MA than that on the contralateral PIA (-11.5% and -2.0%, respectively, p<0.01, Fig. 6). In particular, the decrease was most remarkable in the females operated on via MA (-13.5%, p<0.01). By contrast, the results on the PIA side showed no statistical difference between preoperative and follow-up CT results.
The prevalence of spinal fusion has continued to increase because of the emergence of new techniques with spinal instrumentation and improved imaging modalities that allow for accurate recognition of spinal abnormalities. Inevitably, the paraspinal muscles must be manipulated, and this can lead to iatrogenic injury of the back muscles, causing postoperative muscle atrophy and pain. The purpose of our study was to determine whether PIA for lumbar fusion resulted in less paraspinal muscle atrophy than the traditional MA. We compared paraspinal muscles that were operated on by different approach techniques in the same patient in order to exclude individual clinical condition. The present study measured the multifidus muscle because it is most directly affected by the injury during dissection and retraction. This study was attempted to evaluate the damage to the paraspinal muscle indirectly after MA and PIA by measuring the multifidus muscle volume.
The present study found that the cross-sectional area and thickness of the paraspinal muscles did not change significantly after the surgery on the side of PIA, but decreased remarkably on that of MA, especially in female patients (Fig. 3). Furthermore, the thickness of multifidus muscle was statistically correlated with the cross-sectional area. In most patients, the muscle thickness of the MA showed an early increase up to 8 months after the surgery, then decreased in the follow-up period. Increased paraspinal muscle thickness in the early postoperative stage may be caused by intraoperative damage and reflect continuing intra- or extracellular edema. Decreased paraspinal muscle thickness may become apparent in the late postoperative stage as the edema subsides.
The conventional MA for placement of PS is associated with a high morbidity. The long incisions required extensive deflection of muscle from the spinal processes, and subsequent prolonged wide retraction may result in denervation of the paraspinal musculature (13). Moreover, self-retaining retractors cause a significant rise in intramuscular pressure in the erector spinae muscles, which is maintained throughout the surgical procedure (14). These pressures are sufficient to cause a reduction in capillary perfusion, which may potentially lead to ischemic changes within the muscle, particularly if the retraction time is longer than 2 hr (14-16). The ischemic damage may be the underlying cause for the electrophysiologic and CT abnormalities observed in the paraspinal muscles of these patients, as well as the overall decrease in trunk muscle strength (17). The specific impact of muscle retraction on postoperative low back pain and disability is not well established, but extensor muscle dysfunction caused by paraspinal muscle injury during surgery may play an important role in development of postoperative low back pain (12). We suggest that PSF via PIA has positive effects on repositioning of the paraspinal muscles after PSF.
The results of the current study demonstrate that PSF via PIA causes less paraspinal muscle atrophy than PSF via MA and has positive effects on preservation of the back muscles. The multifidus muscles represent the deepest muscle group in the lumbar region, and the principal action of the multifidus muscle is rotation in the sagittal plane (18, 19). Force exerted by the back muscles stiffens the functional lumbar spinal unit, with the strongest influence coming from the multifidus (20). Previous investigators have reported that dissection and retraction of the paraspinal musculature can lead to denervation and atrophy, which results in an increased risk of failed back surgery syndrome (21, 22). Histologic, enzymatic, and radiologic evidences of back muscle injury in lumbar surgery have been confirmed by several authors (23, 24). Minimally invasive procedures have been developed as a potential solution to this problem. Furthermore, several authors have reported that minimally invasive approach caused less paraspinal muscle damage than traditional approach and had positive effects on postoperative trunk muscle performance (8). Therefore, paraspinal muscles must be carefully manipulated during the operation to improve the results of the lumbar back surgery. Intermittent retraction of the paraspinal muscles and limited use of the electrical cauterizer must be considered. In particular, female patients with preoperative thin back muscles are unlikely to achieve good operative results (1). These patients should be the most carefully treated. Minimally invasive surgery and the PIA with minimal retraction are indicated (25).
There are some limitations in the present study that deserve mention. First, this study was conducted retrospectively by case selection, and was not randomized and controlled. Second, the length of the postoperative follow-up period was not long enough to evaluate the long-term results. Lastly, since the side of MA was based on the location of the preoperative radicular symptoms, there is a possibility of preexisting denervation to paraspinal muscles.
In conclusion, the PIA for lumbar fusion has yielded successful outcomes for the preservation of paraspinal muscle in this series of 26 patients, and further studies on this technique may help define its role as a minimally invasive procedure for spinal fusion. Moreover, future studies with prospective, randomized controlled trials are needed to address issues such as the safety and efficacy of this technique, and whether less muscle atrophy has positive effects on long-term clinical and functional outcome.
Figures and Tables
Fig. 1
The drawing shows the pedicle screw fixation via paramedian interfascial approach to the lumbar spine. IS, interspinalis muscle; LS, longissimus muscle; MF, multifidus muscle; PS, psoas muscle.

Fig. 2
The cross-sectional area, thickness, and width of multifidus muscle were measured on the computed tomography. IS, interspinalis muscle; LS, longissimus muscle; MF, multifidus muscle; PS, psoas muscle.

Fig. 3
Changes of the multifidus muscles on computed tomography in a 57-yr-old woman (A: preoperative; B: follow-up). Note the significant multifidus muscle atrophy on the side of midline approach (B).
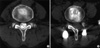
Fig. 4
Box plot showing the postoperative changes of the crosssectional area of multifidus muscle on the side of paramedian interfascial and midline approach. Box plots show the median value (horizontal line in box), and the interquartile range (25-75%) is represented by the box. *p<0.05.

Fig. 5
Postoperative changes in paraspinal muscle thickness. (A) On the side of midline approach, (B) On the side of paramedian interfascial approach.

Fig. 6
Box plot showing the postoperative changes of the thickness of multifidus muscle on the side of paramedian interfascial and midline approach. (A) male patients, (B) female patients. Box plots show the median value (horizontal line in box), and the interquartile range (25-75%) is represented by the box. *p<0.05.

ACKNOWLEDGMENTS
The authors thank Sang-Hwa Lee for his invaluable assistance and technical support and Dr. BoRae G. Park for her excellent advice and support.
References
1. Harris BM, Hilibrand AS, Savas PE, Pellegrino A, Vaccaro AR, Siegler S, Albert TJ. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine. 2004. 29:E65–E70.
2. Suwa H, Hanakita J, Ohshita N, Gotoh K, Matsuoka N, Morizane A. Postoperative changes in paraspinal muscle thickness after various lumbar back surgery procedures. Neurol Med Chir (Tokyo). 2000. 40:151–154.

3. Fritzell P, Hagg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine. 2002. 27:1131–1141.
4. Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002. 15:355–361.

5. Taylor H, McGregor AH, Medhi-Zadeh S, Richards S, Kahn N, Zadeh JA, Hughes SP. The impact of selfretaining retractors on the paraspinal muscles during posterior spinal surgery. Spine. 2002. 27:2758–2762.

6. Vialle R, Court C, Khouri N, Olivier E, Miladi L, Tassin JL, Defives T, Dubousset J. Anatomical study of the paraspinal approach to the lumbar spine. Eur Spine J. 2005. 14:366–371.

7. Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993. 18:575–581.

9. Kim SW, Shin H. Simultaneous paraspinal and midline Approach for upper lumbar disc herniation: technique to prevent lamina fracture. J Korean Neurosurg Soc. 2005. 38:111–115.
10. Harms J, Jeszensky D. The unilateral transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998. 6:88–99.
11. Boelderl A, Daniaux H, Kathrein A, Maurer H. Danger of damaging the medial branches of the posterior rami of spinal nerves during a dorsomedian approach to the spine. Clinical Anatomy. 2002. 15:77–81.

12. Stevens KJ, Spenciner DB, Griffiths KL, Kim KD, Zwienenberg-Lee M, Alamin T, Bammer R. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006. 19:77–86.

13. Fritzell P, Hagg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine. 2002. 27:1131–1141.
14. Styf JR, Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 1998. 23:354–358.

15. Datta G, Gnanalingham KK, Peterson D, Mendoza N, O'Neill K, Van Dellen J, McGregor A, Hughes SP. Back pain and disability after lumbar laminectomy: is there a relationship to muscle retraction? Neurosurgery. 2004. 54:1413–1420.

16. Taylor H, McGregor AH, Medhi-Zadeh S, Richards S, Kahn N, Zadeh JA, Hughes SP. The impact of selfretaining retractors on the paraspinal muscles during posterior spinal surgery. Spine. 2002. 27:2758–2762.

17. Pradhan BB, Nassar JA, Delamarter RB, Wang JC. Single-level lumbar spine fusion: a comparison of anterior and posterior approaches. J Spinal Disord Tech. 2002. 15:355–361.

18. Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech. 1986. 1:205–213.

19. Macintosh JE, Bogduk N. The morphology of the lumbar erector spinae. Spine. 1987. 12:658–668.
20. Wilke HJ, Wolf S, Claes LE, Arand M, Wiesend A. Stability increase of the lumbar spine with different muscle groups: a biomechanical in vitro study. Spine. 1995. 20:192–198.
21. Sihvonen T, Herno A, Paljarvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993. 18:575–581.

22. Weiner BK, Walker M, Brower RS, McCulloch JA. Microdecompression for lumbar spinal canal stenosis. Spine. 1999. 24:2268–2272.

23. Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999. 24:1023–1028.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download