Abstract
The aims of this study were to evaluate the clinicopathologic features of Helicobacter heilmannii-associated gastritis and to compare H. heilmannii-associated gastritis with H. pylori-associated gastritis. We reviewed 5,985 consecutive gastric biopsy specimens. All cases of chronic gastritis with Helicobacter infection were evaluated with the Updated Sydney System, and the grades of all gastritis variables were compared between H. heilmannii-associated gastritis and H. pylori-associated gastritis groups. There were 10 cases of H. heilmannii-associated gastritis (0.17%) and 3,285 cases of H. pylori-associated gastritis (54.9%). The organisms were superficially located within the mucous layer without adhesion to epithelial cells. Interestingly, in one case many intracytoplasmic H. heilmannii organisms were observed in parietal cells with cell damage. A case of low-grade mucosa-associated lymphoid tissue (MALT) lymphoma concomitant with H. heilmannii infection was detected. Compared to H. pylori-associated gastritis, H. heilmannii-associated gastritis showed less severe neutrophilic activity (p<0.0001), mononuclear cell infiltration (p=0.0029), and endoscopic findings of chronic gastritis devoid of erosion or ulcer (p=0.0309). In conclusion, we present the detailed clinicopathologic findings of H. heilmannii-associated gastritis compared to H. pylori-associated gastritis. H. heilmannii-associated gastritis is uncommon and milder than H. pylori-associated gastritis, however it may be noteworthy with respect to the development of MALT lymphoma.
Helicobacter pylori is believed to be one of the most common pathogenic infections in humans, with prevalence rates reaching 60% in adults (1). H. pylori infection is associated with the development of chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (2-4). Helicobacter heilmannii, previously known as Gastrospirillum hominis, has also been described as a possible pathogen producing gastritis in humans (5-12). Its prevalence is much lower than that of H. pylori, ranging from 0.1-2.7% (5, 6, 9, 10, 12). Unlike H. pylori, H. heilmannii has often been found in animals, including primates, dogs, cats, and pigs. It has been suggested that H. heilmannii infection in humans occur through a zoonotic transmission (11). This bacterium has been seen in association with the full spectrum of human gastric diseases like H. pylori as well as H. heilmannii-associated gastritis seems to be less significant than H. pylori-associated gastritis (6, 8, 12). Korea is a country with a high prevalence of H. pylori infection, with prevalence rates reaching 70% (13). However no data about the prevalence of H. heilmannii infection in Korea has been available to date, and there have been a few comparative studies of H. heilmannii-associated gastritis and H. pylori-associated gastritis (8, 10). During the course of two years, ten cases of H. heilmannii gastritis were detected in mucosal biopsies that were submitted for a routine histologic diagnosis.
The aims of this study were to evaluate the clinicopathologic features of H. heilmannii-associated gastritis and to concretely compare between H. heilmannii-associated gastritis and H. pylori-associated gastritis using the Updated Sydney System.
The present study was based on the analysis of 5,985 gastric biopsy specimens from 5,593 consecutive patients with upper gastrointestinal symptoms referred for upper gastrointestinal endoscopy between August 2003 and July 2005. Of the 5,593 patients, 3,417 were men and 2,176 were women (age range: 13-90 yr; mean age, 51 yr). Gastric biopsies were obtained from the areas showing abnormal endoscopic findings. We excluded the cases diagnosed as malignancy, epithelial dysplasia, or specific type of gastritis. There were 10 patients with H. heilmannii infection (0.17%) and 3,285 patients with H. pylori infection (54.9%). We reviewed the clinical and endoscopic findings in all Helicobacter-positive patients.
The gastric biopsy specimens were routinely fixed in 10% buffered formalin. Three serial sections (3 µm thick) were stained with hematoxylin and eosin (H&E) stain for the histologic examination of gastric mucosa. In addition, Wright-Giemsa stain was performed for the detection of Helicobacter including H. heilmannii and H. pylori, and immunohistochemical staining using the rabbit polyclonal anti-H. pylori antibody (B0471, DAKO, Carpinteria, CA, U.S.A.) were done in all cases with H. heilmannii. In agreement with the previous descriptions (5), the morphological criteria for H. heilmannii identification that we used for this study were a predominantly straight appearance, corkscrew-shape spirals, and large size (more than 3 µm in length). The following histologic data were recorded in H. heilmannii-associated gastritis: 1) number of organisms; 2) localization of organisms; 3) associated pathologic findings. In addition, all cases of Helicobacter-associated gastritis were evaluated with the Updated Sydney System (14): 0, absent; 1, mild; 2, moderate; 3, marked, and we compared the graded features of H. heilmannii-associated gastritis to those of H. pylori-associated gastritis.
The clinicopathologic parameters between the H. pylori-associated gastritis and H. heilmannii-associated gastritis groups were examined with the chi-square or Fisher's exact test. To analyze the statistical differences of the average score of graded variables of the Updated Sydney System between two groups, the Mann-Whitney U test was used. Correlation coefficients were evaluated for correlations between Helicobacter density and inflammatory activity by Spearman correlation analysis. Significance was defined as p<0.05. All statistical analyses were performed using SPSS software (version 10.0, SPSS INC., Chicago, IL, U.S.A.).
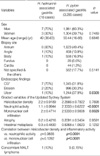
The prevalence of H. heilmannii in our series of gastric biopsies was 0.17% (10 of 5,985), whereas the prevalence of H. pylori was 54.9% (3,285 of 5,985). The patients with H. heilmannii infection consisted of 3 women and 7 men with a mean age of 49 yr (range 36-63 yr). Most patients had mild gastrointestinal symptoms, including epigastric discomfort and nausea. Five (50%) patients had raised a domestic animal in their house (dog, 4 patients; pig, 1 patient). None of the H. heilmannii-infected patients showed a co-infection of H. pylori. All biopsies revealed chronic gastritis with variable numbers of spiral microorganisms, ranging from 3 to over 500. Table 1 details the clinicopathologic findings of the patients with H. heilmannii infection.
Between two groups, there was no significant difference in patient's age and sex, and the lesion sites. Endoscopic findings of 10 patients with H. heilmannii infection were mainly chronic gastritis (n=7), and the others were erosion (n=2) and ulcer (n=1), respectively. In contrast, in H. pylori-associated gastritis group, the endoscopic findings were chronic gastritis (31.8%), erosion (30.3%), and ulcer (37.9%), respectively (Fig. 1). Histologically, the gastric mucosa of the patients with H. heilmannii infection disclosed chronic gastritis similar to H. pylori-associated gastritis (Fig. 2A). We compared the mean scores of all graded features of the Updated Sydney System of H. heilmannii-associated gastritis with those of H. pylori-associated gastritis. Neutrophil activity and mononuclear cell infiltration were significantly lower in H. heilmannii-associated gastritis than in H. pylori-associated gastritis (p<0.0001 and p=0.0029). Significant correlations between Helicobacter density and inflammatory activity were found in the H. pylori-associated gastritis group (vs. neutrophilic activity, p<0.0001; vs. mononuclear cell infiltration, p<0.0001), but not in the H. heilmannii-associated gastritis group (vs. neutrophilic activity, p=0.9835; vs. mononuclear cell infiltration, p=0.1282). Also, adhesion to epithelial cells and surface epithelial damage were infrequent in cases of H. heilmannii-associated gastritis (Fig. 2B, C).
H. heilmannii showed a characteristic corkscrew appearance with more than four spirals (Fig. 2D). These organisms had long (about 4-10 µm), straight, tightly coiled morphology, as compared to H. pylori's typical curved, rod-like or short, spiral morphology. They were readily distinguished from H. pylori in H&E stained sections. They were also stained with Wright-Giemsa and Warthin-Starry stains, but not with Gram stain. Immunostaining with the rabbit polyclonal anti-H. pylori antibody revealed reactivity with H. heilmannii as well (Fig. 2D, inset). They were predominantly found both in the gastric foveolar lumen and on the mucous layer without adhesion to epithelial cells. Interestingly, in one case many spiral organisms were observed within the cytoplasm of the parietal cells, up to 10 in number per one parietal cell (Fig. 2E). Most intracytoplasmic organisms were present in the vacuole-like clear spaces and were morphologically intact. Some parietal cells harboring the organisms showed swollen and degenerated appearance with polymorphonuclear cell infiltration (Fig. 2F). Meanwhile, no intracytoplasmic H. pylori was noted throughout not only parietal cells but also foveolar epithelial cells.
Lymphoid follicles were noted in two cases of H. heilmannii-associated gastritis, one of which showed low-grade MALT lymphoma. Of our series, one case of low-grade MALT lymphoma concomitant with H. heilmannii infection was present, whereas 5 cases of low-grade MALT lymphoma were found in 3,285 cases of H. pylori-associated gastritis (0.15%). Histologically, biopsies revealed the classical histologic features of low-grade MALT lymphoma, such as mucosal proliferation of centrocyte-like cells with plasma cell differentiation, hyperplastic lymphoid follicles, and lymphoepithelial lesions (LELs) (Fig. 3A-C). The lymphoma cells were positive for CD20, Bcl-2 but negative for CD3, CD5, CD10, and cyclin D1, consistent with MALT lymphoma. H. heilmannii was abundant in the mucous layer or on the surface of the adjacent foveolar epithelia, but no H. pylori was identified (Fig. 3D). Later, the patient was treated with the same regimen of H. pylori eradication therapy, resulting in the complete disappearance of lymphoma cells. The patient showed no sign of recurrence endoscopically and histologically, as of 18 months after the eradication treatment.
H. pylori is the most common gastric-colonizing bacterium found in humans that causes various gastroduodenal diseases (1, 2-4). H. heilmannii, a Helicobacter species found in the stomach of domestic animals, is another pathogen producing chronic gastritis in human (5-12). In this study, ten cases of H. heilmannii infection were detected out of 5,593 symptomatic subjects undergoing upper gastrointestinal endoscopy (a prevalence of 0.17%), whereas H. pylori was found in 54.9% of the same 5,593 patients. Our results are in general agreement with the reported prevalences of these organisms (5, 6, 9, 10, 12). Until now, only one case of H. heilmannii-associated gastritis in the human stomach has been reported in Korean population (15). To the best of our knowledge, this report is the first Korean series of H. heilmannii-associated gastritis in human stomach. Morphologically, it is not difficult to differentiate H. heilmannii from the other non-spiral gastric organisms including H. pylori. However, as a variety of large gastric spiral organisms such as H. felis, H. salomonis, and H. bizzozeronii, although very rarely identified in human stomach, are indistinguishable from H. heilmannii on routine light microscopy, 16S rDNA sequencing or fluorescence in situ hybridization (FISH) with specific probes are required for more definitive identification (16).
In the present study, all patients with H. heilmanni infection showed chronic gastritis similar to H. pylori-associated gastritis. However, the neutrophilic activity and mononuclear cell infiltration were significantly low in cases of H. heilmannii-associated gastritis compared to H. pylori-associated gastritis. Moreover, no correlation between Helicobacter density and inflammatory activity was found in the H. heilmannii-associated gastritis group in contrast to the H. pylori-associated gastritis group. The neutrophilic infiltration has been regarded to represent the activity of H. pylori gastritis with a close relation to the Helicobacter density, which also plays an important role in epithelial damage (17); however, such findings were not evident in H. heilmannii-associated gastritis. It has been described that H. heilmannii does not predominantly contact with gastric epithelial cells in contrast to H. pylori (12), as seen in our cases: these different localization of H. heilmannii may be related with mild neutrophilic infiltration. Taken together, H. heilmannii-associated gastritis is generally milder than H. pylori-associated gastritis, and H. heilmannii seems less likely to attract inflammatory cells, especially neutrophils, than H. pylori. It is worthwhile that the present study provides the concretely comparative data of H. heilmannii-associated gastritis and H. pylori-associated gastritis using the Updated Sydney System.
The distinctive histologic features of H. heilmannii-associated gastritis mentioned by the previous reports included the followings; absence of epithelial damage (7), lymphocytic exudation into gastric foveolae (9), mild inflammatory activity (8, 10), infrequent acquired MALT (8, 10), and infrequent intestinal metaplasia (8). In the present study, mild inflammatory activity and less prominent foveolar epithelial damages were confirmed, but the other findings were inconspicuous. Of this series of H. heilmannii-associated gastritis, one case of erosive gastritis showed intracytoplasmic organisms in parietal cells. Dubois et al. reported that Gastrospirillum hominis-like organisms (GHLO) frequently invaded and damaged parietal cells of monkeys (18). Up to the present time, we could find only three reports describing intracellular spiral organisms in human parietal cells (5, 19, 20). In our case, many H. heilmannii organisms invaded into parietal cells accompanied by cellular changes. Although a direct invasion into parietal cells of H. heilmannii has been rarely observed, it seems to be one of the characteristic histologic findings of H. heilmannii-associated gastritis.
The association between H. pylori and gastric MALT lymphoma is well established: H. pylori can be demonstrated in the gastric mucosa of most cases of gastric MALT lymphoma (3, 4, 21). O'Rourke et al. have shown that up to 25% of H. heilmannii-infected mice develop gastric MALT lymphomas (22). However, H. heilmannii-associated MALT lymphoma has been rarely reported in human: Less than 20 cases have been listed in Medline (Pubmed) (8, 23-25), including the recently additional 4 cases reported by Okiyama et al. (12). Herein, we present one additional case of H. heilmannii-associated MALT lymphoma. This patient was well treated with conventional Helicobacter eradication, and repeated histologic examination showed neither tumor recurrence nor Helicobacter infection during one and a half year-follow up.
In conclusion, our study provides the detailed clinicopathologic characteristics of H. heilmannii-associated gastritis as well as its prevalence firstly reported in Koreans. Even though H. heilmannii-associated gastritis is uncommon and milder than H. pylori-associated gastritis, it may be noteworthy with respect to the development of MALT lymphoma. Thus, the better understandings of the diagnostic morphologies and clinical significance of H. heilmannii infection in human stomach by the pathologists and the clinicians may be necessary.
Figures and Tables
Fig. 1
Gastrofiberscopic findings. (A) An active ulcer is noted in the body of the patient with H. pylori-associated gastritis. (B) Mild mucosal hyperemia is noted in the antrum of the patient with H. heilmannii-associated gastritis.
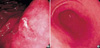
Fig. 2
H. heilmannii-associated gastritis. (A) The antral mucosa shows mild chronic gastritis with minimal neutrophilic activity (hematoxylineosin, ×100). (B) Neutrophilic infiltration and surface epithelial changes are mild in H. heilmannii-associated gastritis with high Helicobacter density, in contrast to H. pylori-associated gastritis showing dense neutrophilic infiltration into the epithelia and surface epithelial damage (C) (hematoxylin-eosin, ×400). (D) Many corkscrew-shaped spiral organisms are noted within the mucous adjacent to the gastric foveolar epithelia (hematoxylin-eosin, ×1,000). They show a cross-reaction for H. pylori immunostaining (inset). (E) Spiral organisms within a parietal cell demonstrate intact morphology and surrounding vacuole-like spaces (hematoxylin-eosin, ×1,000). (F) Parietal cells harboring organisms show degenerative change with neutrophilic infiltration (hematoxylin-eosin, ×1,000).
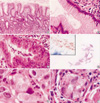
Fig. 3
H. heilmannii-associated gastric MALT lymphoma. (A) Low magnification view reveals extensive lymphocytic infiltration (hematoxylineosin, ×100) with an asterisk indicating areas of (B) and (C), and an inset representing (D) area. (B) Lymphomatous infiltrations form destructive lymphoepithelial lesions (LELs) (hematoxylin-eosin, ×400). (C) Cytokeratin immunostain highlights the remaining epithelial remnant in LELs (×400). (D) Many spiral organisms are present in the adjacent gastric pit of this case (Giemsa stain, ×1,000).
References
1. Marshall BJ. Helicobacter pylori. Am J Gastroenterol. 1994. 89:116–128.
2. Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997. 11:71–88.
3. Nakamura S, Yao T, Aoyagi K, Iida M, Fujishima M, Tsuneyoshi M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer. 1997. 79:3–11.
5. Heilmann KL, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991. 32:137–140.
6. Hilzenrat N, Lamoureux E, Weintrub I, Alpert E, Lichter M, Alpert L. Helicobacter heilmannii-like spiral bacteria in gastric mucosal biopsies. Prevalence and clinical significance. Arch Pathol Lab Med. 1995. 119:1149–1153.
7. Holck S, Ingeholm P, Blom J, Norgaard A, Elsborg L, Adamsen S, Andersen LP. The histopathology of human gastric mucosa inhabited by Helicobacter heilmannii-like (Gastrospirillum hominis) organisms, including the first culturable case. APMIS. 1997. 105:746–756.
8. Stolte M, Kroher G, Meining A, Morgner A, Bayerdorffer E, Bethke B. A comparison of Helicobacter pylori and Helicobacter heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 1997. 32:28–33.
9. Ierardi E, Monno RA, Gentile A, Francavilla R, Burattini O, Marangi S, Pollice L, Francavilla A. Helicobacter heilmannii gastritis: a histological and immunohistochemical trait. J Clin Pathol. 2001. 54:774–777.
10. Jhala D, Jhala N, Lechago J, Haber M. Helicobacter heilmannii gastritis: association with acid peptic diseases and comparison with Helicobacter pylori gastritis. Mod Pathol. 1999. 12:534–538.
11. Stolte M, Wellens E, Bethke B, Ritter M, Eidt H. Helicobacter heilmannii (formerly Gastrospirillum hominis) gastritis: an infection transmitted by animals? Scand J Gastroenterol. 1994. 29:1061–1064.
12. Okiyama Y, Matsuzawa K, Hidaka E, Sano K, Akamatsu T, Ota H. Helicobcater heilmannii infection: Clinical, endoscopic and histological features in Japanese patients. Pathol Int. 2005. 55:398–404.
13. Lee YE, Kim HS, Lee SK, Lee HT, Lee YJ, Bae CY. Prevalence and risk factors of Helicobacter pylori infection in general population. J Korean Acad Fam Med. 1999. 20:186–193.
14. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996. 20:1161–1181.
15. Seo WJ, Park CS, Cho YJ, Cha KW, Lee SW, Lim ST, Sung YH, Baek AR. A case of gastric ulcer induced by Helicobacter heilmannii-like organism. Korean J Gastroenterol. 2003. 42:63–66.
16. Priestnall SL, Wiinberg B, Spohr A, Neuhaus B, Kuffer M, Wiedmann M, Simpson KW. Evaluation of "Helicobacter heilmannii" subtypes in the gastric mucosas of cats and dogs. J Clin Microbiol. 2004. 42:2144–2151.
17. Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991. 32:1415–1418.
18. Dubois A, Tarnawski A, Newell DG, Fiala N, Dabros W, Stachura J, Krivan H, Heman-Ackah LM. Gastric injury and invasion of parietal cells by spiral bacteria in rhesus monkeys. Are gastritis and hyperchlorhydria infectious diseases? Gastroenterology. 1991. 100:884–891.
19. Dye KR, Marshall BJ, Frierson HF Jr, Guerrant RL, McCallum RW. Ultrastructure of another spiral organism associated with human gastritis. Dig Dis Sci. 1989. 34:1787–1791.

20. Rollason TP, Stone J, Rhodes JM. Spiral organisms in endoscopic biopsies of the human stomach. J Clin Pathol. 1984. 37:23–26.

21. Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991. 338:1175–1176.
22. O'Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of "Helicobacter heilmannii" infection. J Pathol. 2004. 203:896–903.
23. Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdorffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000. 118:821–828.
24. Thomas-Marques L, Yaziji N, Bouche O, Diebold MD, Cadiot G, Thiefin G. Helicobacter heilmannii-associated low-grade gastric MALT lymphoma: a new case of complete remission after eradication. Gastroenterol Clin Biol. 2005. 29:476–477.
25. Regimbeau C, Karsenti D, Durand V, D'Alteroche L, Copie-Bergman C, Metman EH, Machet MC. Low-grade gastric MALT lymphoma and Helicobacter heilmannii (Gastrospirillum hominis). Gastroenterol Clin Biol. 1998. 22:720–723.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download