Abstract
The distinction between benign and malignant thyroid tumors is critical for the management of patients with thyroid nodules. We applied immunohistochemical staining for galectin-3, HBME-1, cytokeratin 19 (CK19), high molecular weight cytokeratin (HMWCK), cyclin D1 and p27kip1 in 295 thyroid lesions to determine their diagnostic accuracy. The expression of all markers was significantly associated with differentiated thyroid carcinoma (DTC).The sensitivity for the diagnosis of DTC was 94.7% with galectin-3, 91.3% with HBME-1, and 90.3% with CK19. The specificities of these markers were 95.5%, 69.7%, and 83.1%, respectively. Combining these markers, co-expression of galectin-3 and CK19 or galectin-3 and HBME-1 was seen in 93.2% of carcinomas but in none of the benign nodules. Comparing follicular variant of papillary carcinoma (FVPC) with follicular carcinoma (FC), the expression of galectin-3, CK19, and HMWCK was significantly higher in FVPC. When comparing FC with FA, the expression of galectin-3 and HBME-1 was significantly higher in FC. These results suggest that 1) galectin-3 is a useful marker in the distinction between benign and malignant thyroid tumors, 2) the combined use of HBME-1 and CK19 can increase the diagnostic accuracy, and 3) the use of CK19 and HMWCK can aid in the differential diagnosis between PC and FC.
The current diagnostic method of surgically resected thyroid nodules is pathologic evaluation of routine hematoxylin and eosin stained slides. For example, the diagnosis of papillary thyroid carcinoma is based on the presence of papillary architecture and characteristic nuclear features, such as ground glass nuclei, nuclear pseudoinclusions, and nuclear grooves (1). In cases of follicular variant of papillary carcinoma, the pathologic diagnosis depends on the typical nuclear changes of papillary thyroid carcinoma. However, the interpretation of nuclear features may be quite subjective and interobserver disagreements among pathologists are well documented (2, 3). The diagnosis of encapsulated follicular lesions is also quite problematic, especially when minor nuclear changes seen in typical papillary carcinoma are found (3). However, an accurate diagnosis is crucial for the appropriate treatment and long-term management of tumors, and thus many immunohistochemical markers have been suggested to aid in the differential diagnosis of thyroid nodules.
Galectin-3 is a member of a growing family of β-galactoside-binding animal lectins, involved in the regulation of cell-cell and cell-matrix interaction, cell growth, neoplastic transformation, and apoptosis (4). Many studies have found that galectin-3 expression is of value in discriminating benign and malignant thyroid nodules (5-13), although a few reports contradict the results (14, 15). Generally, it has been suggested as a marker of thyroid malignancy with relatively high sensitivity and specificity, especially in papillary carcinoma (5-13).
HBME-1 is a monoclonal antibody generated against an unknown membrane antigen of mesothelial cells (16). It was also reported to show preferential reactivity with malignant thyroid tumors (11, 12, 17-19).
The differential expression of cytokeratins has been evaluated in various thyroid lesions. Among various cytokeratins, cytokeratin 19 (CK19) has been reported to be useful in the diagnosis of papillary carcinoma, where it has shown diffuse and strong cytoplasmic staining (11-13, 20-22). The expression of high molecular weight cytokeratin (HMWCK), although patchy, has also been found to be significantly increased in papillary thyroid carcinoma and has been reported to be helpful in distinguishing it from other benign and malignant thyroid nodules (20, 23).
The loss of regulatory control of the cell cycle, leading to unrestrained cell proliferation, is a hallmark of cancers. Cyclin D1 plays a key role in the regulation of the G1/S transition through the cell cycle. Although little is known about the role of cyclin D1 in the pathogenesis of thyroid carcinoma, its overexpression has been reported in malignant thyroid tumors (24-26). p27kip1, a cyclin-dependent kinase inhibitory protein, is down-regulated in thyroid carcinoma and reported to be useful in the differential diagnosis of papillary hyperplasia in Grave's disease and papillary thyroid carcinoma (25, 27-29).
Most studies have evaluated the single expression of markers in various thyroid lesions and a few reports have studied the combined expression of markers (11, 12, 18). In this study, we compared the single or combined expression of galectin-3, HBME-1, CK19, HMWCK, cyclin D1 and p27kip1 in a large number of benign and malignant thyroid lesions to determine their diagnostic accuracy in the differential diagnosis of thyroid nodules.
Two hundred forty-one consecutive cases of thyroid tumors including 181 papillary carcinomas, 25 follicular carcinomas, and 35 follicular adenomas were collected from Seoul National University Bundang Hospital from May 2003 to April 2005. The papillary carcinomas included 76 classic, 17 follicular and 4 tall cell variants, and 84 microcarcinomas. The follicular carcinoma cases included 3 metastatic follicular carcinomas in the lung or vertebra, 2 widely invasive follicular carcinomas, and 20 minimally invasive follicular carcinomas. Minimally invasive follicular carcinomas were diagnosed by the presence of definite capsular and/or vascular invasion. We also included 54 nodular hyperplasias to study the expression of markers in non-neoplastic thyroid nodules. All cases were reviewed and confirmed by an endocrine pathologist.
All specimens were fixed in 10% buffered formalin, embedded in paraffin wax, and stained with hematoxylin and eosin (H-E) for histologic examination. Representative paraffin blocks selected for immunohistochemistry included both the tumor and the normal thyroid tissue.
Four micron-thick sections were deparaffinized, rehydrated in graded alcohols, and processed using DAKO envision detection kit (DakoCytomation, Carpinteria, CA, U.S.A.). Briefly, antigen retrieval was performed in a microwave oven for 15 min in 10 mM citrate buffer pH 6.0. Endogenous peroxidase activity was blocked with a 3% H2O2-methanol solution, and the slides were incubated in 10% normal goat serum for 30 min to prevent non-specific staining. They were then incubated for 1 hr at room temperature with an appropriately diluted primary antibody. The following mouse monoclonal antibodies were used: galectin-3 (clone 9C4; 1:600; Novocastra, Newcastle, U.K.), HBME-1 (clone HBME-1; 1:100; DakoCytomation, Carpinteria, CA, U.S.A.), CK19 (clone RCK108; 1:150; DakoCytomation), HMWCK (clone 34βE12; 1:150; DakoCytomation), cyclin D1 (clone SP4; 1:100; Labvision, Fremont, CA, U.S.A.), p27kip1 (clone SX-53G8; 1:200; DakoCytomation). Thereafter, the sections were incubated with DAKO Envision/HRP for 30 min. Diaminobenzidine was used as a chromogen, and the sections were counterstained with Mayer's hematoxylin. As a negative control, non-immune serum was substituted for the primary antibody.
Galectin-3 expression was both cytoplasmic and/or nuclear. HBME-1 was expressed in the cytoplasm and cell membrane with occasional luminal accentuation. CK19 and HMWCK expressions were cytoplasmic with membranous accentuation. Cyclin D1 and p27kip1 were mostly expressed in the nucleus with occasional cytoplasmic staining and only nuclear staining was counted.
The expression of markers except for p27kip1 was assessed as follows: 0, no staining or staining in less than 10% of the tumor cells; 1+, staining in 10% to 25% of the cells; 2+, staining in 26% to 50% of the cells; 3+, staining in 51% to 75% of the cells; 4+, staining in more than 75% of the cells. Staining of 1+ or 2+ was defined as focal staining, and staining of 3+ or more was defined as diffuse staining. For statistical analysis, cases with any degree of positive staining were grouped as positive. For p27kip1, no staining or staining in less than 10% of the tumor cells were defined as loss of p27kip1.
Statistical analysis was performed with SPSS software (version 11.0, SPSS Inc., Chicago, IL, U.S.A.). Chi-square or Fisher's exact tests were used when comparing frequencies between groups. The sensitivity, specificity, and accuracy of the markers and their combination in the diagnosis of differentiated thyroid carcinomas (DTC) including both papillary and follicular carcinomas were compared. Probability values less than 0.05 were considered statistically significant.
The expression of galectin-3, HBME-1, CK19, HMWCK, cyclin D1, and p27kip1 in various thyroid nodules is shown in Table 1. The overexpression of all markers except p27kip1 and the loss of p27kip1 were significantly associated with DTC (p<0.001 in all markers).
The sensitivity, specificity, and diagnostic accuracy of the markers for the diagnosis of DTC are summarized in Table 2. Galectin-3, HBME-1, and CK19 expressions were highly sensitive, but HMWCK and cyclin D1 expression and loss of p27kip1 showed intermediate or low sensitivity. The expression of galectin-3 or HMWCK was highly specific for DTC. The expression of galectin-3 in the benign nodule was only 4.5% (4/89) and HMWCK was not detected in any of the 89 benign nodules.
From these results, we concluded that galectin-3 is the most useful marker for the detection of DTC, and we subanalyzed the individual cases showing false-positive or false-negative staining for galectin-3.
Four benign nodules showed galectin-3 expression, one case being follicular adenoma and three cases being nodular hyperplasia. Two cases of nodular hyperplasia exhibited benign papillary hyperplasia. All the cases showed focal and weak staining for galectin-3, and there was no expression of HBME-1 or CK19 (Fig. 1).
On the contrary, 11 malignant tumors were negative for galectin-3. One case was a follicular variant of papillary carcinoma, another was a papillary microcarcinoma with follicular pattern and the others were follicular carcinomas. One of the galectin-3-negative follicular carcinomas was a metastatic tumor in the spine. All these cases showed positive staining for HBME-1, and eight of the cases were positive for CK19.
To improve the diagnostic accuracy of galectin-3, we analyzed the combined effect of other sensitive markers, HBME-1 or CK19 (Table 2). The expression of galectin-3 or HBME-1 was noted in 100% of carcinomas and 34.8% of benign nodules (p<0.001). The expression of galectin-3 or CK19 was detected 98.5% of carcinomas and 21.3% of benign nodules (p<0.001). When we analyzed the co-expression of galectin-3 and HBME-1 or galectin-3 and CK19, the specificity was improved up to 100%, but the sensitivity was not higher than that of galectin-3 alone.
When comparing papillary carcinoma and follicular carcinoma, the expression of galectin-3, CK19, HMWCK, cyclin D1 and the loss of p27kip1 was significantly higher in papillary carcinoma than in follicular carcinoma (p<0.001; Table 1, Fig. 2). HBME-1 was highly expressed in both papillary and follicular carcinoma (91.7% and 88.0%, respectively). When comparing follicular variant of papillary carcinoma with follicular carcinoma, the expression of galectin-3 (p=0.031), CK19 (p<0.001) and HMWCK (p=0.002) were significantly higher in follicular variant of papillary carcinoma than in follicular carcinoma (Table 3). The expression of HBME-1 and cyclin D1 and the loss of p27kip1 were not significantly different between the two groups.
We also evaluated the differences in immunohistochemical findings in the variants of papillary carcinoma. When comparing follicular variant of papillary carcinoma with classic papillary carcinoma, the expression of HMWCK (p=0.008) was significantly lower in follicular variant of papillary carcinoma than in classic papillary carcinoma (Table 3). No significant differences were found between follicular variant of papillary carcinoma and classic papillary carcinoma for the expression of other markers except for HMWCK.
The expression of galectin-3 and HBME-1 was significantly higher in follicular carcinomas than in follicular adenomas (Table 1, Fig. 3). Galectin-3 was detected in 64.0% of follicular carcinomas and 2.9% of follicular adenomas (p<0.001). HBME-1 was expressed in 88% of follicular carcinomas and 48.6% of follicular adenomas (p=0.0017). The expression of CK19, HMWCK, cyclin D1 and p27 were not significantly different between follicular carcinoma and adenoma (Table 1). CK19 was expressed in 44.0% of follicular carcinomas and 28.6% of follicular adenomas.
In this study, we evaluated the diagnostic accuracy of galectin-3, HBME-1, CK19, HMWCK, cyclin D1, and p27kip1 in the differential diagnosis of thyroid nodules. We found that galectin-3, HBME-1, and CK19 were highly sensitive for DTC, but galectin-3 was the most sensitive and specific.
Galectin-3 has been suggested to be a highly sensitive and reliable diagnostic marker for the preoperative identification of thyroid carcinomas (5, 6, 8). In a large multicenter study by Bartolazzi et al. (8), the sensitivity and the specificity of galectin-3 immunodetection in the differential diagnosis of benign and malignant thyroid lesions were higher than 99% and 98%, respectively. Although galectin-3 has been consistently suggested to be a very sensitive marker for papillary carcinoma (5-13), some studies revealed that it shows low reactivity in follicular carcinoma (7, 9, 12). Our study also showed that the expression of galectin-3 was significantly higher in papillary carcinomas than in follicular carcinomas (98.9% vs. 64.0%; p<0.001). In addition, whereas most of the papillary carcinomas showed diffuse and strong positivity to galectin-3, follicular carcinomas showed focal positivity in 9 (56%) of 16 galectin-3 positive cases, especially in the subcapsular area. Thus, the use of galectin-3 immunohistochemistry in aspiration cytology samples may result in erroneously negative results.
Some recent studies also demonstrated that galectin-3 is highly expressed in benign thyroid lesions and in normal thyroid tissue (11, 14, 15). The discrepancies in the frequency of galectin-3 immunoreactivity in benign lesions may be related to the different antibody detection systems and the cut-off values for positive and negative staining. In the thyroid gland, endogenous biotin is invariably expressed in thyrocytes, mostly in Hurthle cells. Thus, a biotin-based detection system may provide false positive results. It has been suggested that galectin-3 immunodetection may be a useful adjunct in the distinction between benign and malignant thyroid tumors, only if performed in a biotin-free detection system (9, 10).
Besides galectin-3, several markers have been investigated as useful markers for the diagnosis of thyroid carcinoma. HBME-1 has been reported to be one of the most promising markers (11, 12, 17-19). HBME-1 seems to be a sensitive marker for thyroid carcinoma, especially in follicular carcinoma. In our study, it was expressed in 88% of follicular carcinomas, compared with the 64% positivity for galectin-3. However, HBME-1 was also expressed in 48.6% of follicular adenomas and 20.4% of nodular hyperplasias, these frequencies being similar or slightly higher than those previously reported (12, 17, 19). Thus, although HBME-1 contributes to the diagnosis of DTC, it cannot be applied in the preoperative or differential diagnosis of follicular-patterned lesions due to its low specificity.
CK19 has been reported to be strongly and diffusely expressed in papillary carcinoma, whereas it is usually absent or focally expressed in follicular carcinoma and benign nodules (11-13, 20). In our cases, almost all papillary carcinomas including follicular variants of papillary carcinoma showed strong and diffuse CK19 immunoreactivity. However, our nodular hyperplasias, follicular adenomas, and follicular carcinomas also showed CK19 immunoreactivity in 9.3%, 28.6%, and 44.0% of cases, respectively, and 20% of follicular carcinomas and 11% of follicular adenomas showed diffuse staining for CK19, although the staining was less intense than that observed in the papillary carcinoma. It is hard to make any generalizations about the expression of CK19 in follicular lesions due to the relative paucity of cases studied, as most of the previous studies on CK19 expression in thyroid nodules have targeted papillary carcinomas, and the extent of CK19 immunoreactivity in follicular lesions in the previous studies is quite variable. The CK19 immunoreactivity of follicular adenoma in our study was higher than that previously reported. However, Sahoo et al. (22) reported that 25% of their follicular adenomas showed extensive immunoreactivity for CK19 (2+ in 1, 3+ in 4 of 20 follicular adenomas), and Miettinen et al. (21) also reported that 24% of follicular adenomas and 59% of follicular carcinomas showed CK19 reactivity in >10% of lesions, suggesting that CK19 patterns are not reliable in the differentiation between papillary carcinomas and follicular neoplasms. Thus, although the immunoreactivity for CK19 is more frequent and more diffuse in papillary carcinoma, its immunoreactivity in follicular lesions may limit its utility as a diagnostic marker. HMWCK expression was patchy but strong in 59.7% of papillary carcinomas, and its staining was less frequent in follicular variants of papillary carcinoma, being expressed in 35.3%. HMWCK seems to be a highly specific marker for papillary carcinoma; however, its low sensitivity limits its use in diagnosis.
We attempted to identify a combination of markers with the greatest diagnostic accuracy. Nikiforova et al. (30) reported that follicular carcinoma with PAX-PPARγ rearrangement showed immunoreactivity for galectin-3 but not for HBME-1 and that those with RAS mutations displayed HBME-1-positive/galectin-3-negative immunophenotype. Thus, we thought that HBME-1 would complement the low sensitivity of galectin-3 in the diagnosis of follicular carcinoma, and found that all carcinomas were positive for either galectin-3 or HBME-1. An immunohistochemical diagnostic panel comprising these markers increased the sensitivity for DTC to 100%. However, its specificity for DTC was lowered to 65.2%. When we examined the co-expression of galectin-3 and HBME-1 or galectin-3 and CK19 as a possible marker of DTC, we found that it was the most specific and accurate marker with 100% specificity and 95.3% diagnostic accuracy. Although this combined use of HBME-1 and CK19 with galectin-3 cannot increase the sensitivity in the diagnosis of DTC, it can discriminate galectin-3-positive benign lesions from galectin-3-positive thyroid carcinomas and thus increase the specificity.
In our study, the overexpression of cyclin D1 and loss of p27kip1 were associated with DTC. Their expression was much higher in papillary carcinomas than in follicular carcinomas, and there was no difference in expression between follicular carcinomas and follicular adenomas. There have been a few reports demonstrating that cyclin D1 and p27kip1 immunostaining varies according to tumor phenotype (24-28). However, in our study, their sensitivity and specificity for DTC or papillary carcinoma was relatively lower than for the other markers and thus, were not useful in distinguishing between benign and malignant thyroid nodules or between papillary carcinoma and follicular carcinoma.
In summary, galectin-3 is a useful marker in the distinction of benign and malignant thyroid tumors, and the combined use of HBME-1 and CK19 can increase the specificity and diagnostic accuracy. Galectin-3 and HBME-1 can be used as adjuncts for the differential diagnosis of follicular neoplasms, although the low sensitivity of galectin-3 and the low specificity of HBME-1 for the diagnosis of follicular carcinoma should be born in mind. In addition, CK19 and HMWCK can aid in the diagnosis of papillary carcinoma; however, the low specificity of CK19 and low sensitivity of HMWCK may limit their utility.
Figures and Tables
Fig. 1
Benign papillary hyperplasia in nodular hyperplasia showing focal expression of galectin-3 (A) and no expression of HBME-1 (B).
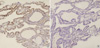
Fig. 2
Papillary carcinoma showing diffuse expression of galectin-3 (A), HBME-1 (B), CK19 (C), and cyclin D1 (D).
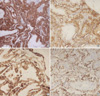
Fig. 3
Expression of galectin-3 and HBME-1 in follicular neoplasm. Follicular carcinoma showing focal expression of galectin-3 (A) and diffuse expression of HBME-1 (B) in area of capsular invasion. Follicular adenoma showing no expression of galectin-3 (C) and focal expression of HBME-1 (D).

Table 1
Expression of the markers in differentiated thyroid carcinoma (DTC) and benign thyroid nodule

Table 2
Sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the immunohistochemical markers in the diagnosis of differentiated thyroid carcinoma

References
1. Rosai J, Carcangiu ML, DeLellis RA. Rosai J, Sobin LH, editors. Tumors of the thyroid gland. Atlas of tumor pathology. 1992. Washington, DC: Armed Forces Institute of Pathology;3rd series. Fasc. 5.
2. Saxen E, Franssila K, Bjarnason O, Normann T, Ringertz N. Observer variation in histologic classification of thyroid cancer. Acta Pathol Microbiol Scand [A]. 1978. 86A:483–486.
3. Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, Tsujimoto M, Kakudo K. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002. 26:1508–1514.

4. Krzeslak A, Lipinska A. Galectin-3 as a multifunctional protein. Cell Mol Biol Lett. 2004. 9:305–328.
5. Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, Cappia S, De Giuli P, Angeli A. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998. 58:3015–3020.
6. Gasbarri A, Martegani MP, Del Prete F, Lucante T, Natali PG, Bartolazzi A. Galectin-3 and CD44v6 isoforms in the preoperative evaluation of thyroid nodules. J Clin Oncol. 1999. 17:3494–3502.

7. Kawachi K, Matsushita Y, Yonezawa S, Nakano S, Shirao K, Natsugoe S, Sueyoshi K, Aikou T, Sato E. Galectin-3 expression in various thyroid neoplasms and its possible role in metastasis formation. Hum Pathol. 2000. 31:428–433.

8. Bartolazzi A, Gasbarri A, Papotti M, Bussolati G, Lucante T, Khan A, Inohara H, Marandino F, Orlandi F, Nardi F, Vecchione A, Tecce R, Larsson O. Thyroid Cancer Study Group. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet. 2001. 357:1644–1650.

9. Herrmann ME, LiVolsi VA, Pasha TL, Roberts SA, Wojcik EM, Baloch ZW. Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med. 2002. 126:710–713.

10. Volante M, Bozzalla-Cassione F, Orlandi F, Papotti M. Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch. 2004. 444:309–312.

11. Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005. 18:48–57.

12. de Matos PS, Ferreira AP, de Oliveira Facuri F, Assumpcao LV, Metze K, Ward LS. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology. 2005. 47:391–401.

13. Park MI, Kang DY. Usefulness of galectin-3, cytokeratin 19, p53, and Ki-67 for the differential diagnosis of thyroid tumors. Korean J Pathol. 2006. 40:86–92.
14. Martins L, Matsuo SE, Ebina KN, Kulcsar MA, Friguglietti CU, Kimura ET. Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J Clin Endocrinol Metab. 2002. 87:4806–4810.

15. Mehrotra P, Okpokam A, Bouhaidar R, Johnson SJ, Wilson JA, Davies BR, Lennard TW. Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology. 2004. 45:493–500.

16. Sheibani K, Esteban JM, Bailey A, Battifora H, Weiss LM. Immunopathologic and molecular studies as an aid to the diagnosis of malignant mesothelioma. Hum Pathol. 1992. 23:107–116.

17. Miettinen M, Karkkainen P. Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity with malignant tumours. Virchows Arch. 1996. 429:213–219.
18. Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod Pathol. 2001. 14:338–342.

19. Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, Tanaka Y, Nakao A. HBME-1 immunostaining in thyroid tumors especially in follicular neoplasm. Endocr J. 2003. 50:173–177.

20. Raphael SJ, McKeown-Eyssen G, Asa SL. High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod Pathol. 1994. 7:295–300.
21. Miettinen M, Kovatich AJ, Karkkainen P. Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch. 1997. 431:407–413.
22. Sahoo S, Hoda SA, Rosai J, DeLellis RA. Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma: a note of caution. Am J Clin Pathol. 2001. 116:696–702.
23. Liberman E, Weidner N. Papillary and follicular neoplasms of the thyroid gland. Differential immunohistochemical staining with high-molecular-weight keratin and involucrin. Appl Immunohistochem Mol Morphol. 2000. 8:42–48.
24. Lazzereschi D, Sambuco L, Carnovale Scalzo C, Ranieri A, Mincione G, Nardi F, Colletta G. Cyclin D1 and Cyclin E expression in malignant thyroid cells and in human thyroid carcinomas. Int J Cancer. 1998. 76:806–811.

25. Wang S, Wuu J, Savas L, Patwardhan N, Khan A. The role of cell cycle regulatory proteins, cyclin D1, cyclin E, and p27 in thyroid carcinogenesis. Hum Pathol. 1998. 29:1304–1309.

26. Goto A, Sakamoto A, Machinami R. An immunohistochemical analysis of cyclin D1, p53, and p21waf1/cip1 proteins in tumors originating from the follicular epithelium of the thyroid gland. Pathol Res Pract. 2001. 197:217–222.
27. Erickson LA, Jin L, Wollan PC, Thompson GB, van Heerden J, Lloyd RV. Expression of p27kip1 and Ki-67 in benign and malignant thyroid tumors. Mod Pathol. 1998. 11:169–174.
28. Resnick MB, Schacter P, Finkelstein Y, Kellner Y, Cohen O. Immunohistochemical analysis of p27/kip1 expression in thyroid carcinoma. Mod Pathol. 1998. 11:735–739.
29. Erickson LA, Yousef OM, Jin L, Lohse CM, Pankratz VS, Lloyd RV. p27kip1 expression distinguishes papillary hyperplasia in Graves' disease from papillary thyroid carcinoma. Mod Pathol. 2000. 13:1014–1019.

30. Nikiforova MN, Lynch RA, Biddinger PW, Alexander EK, Dorn GW 2nd, Tallini G, Kroll TG, Nikiforov YE. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003. 88:2318–2326.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download