Abstract
Extravasation injuries in the neonatal intensive care unit are not rare during parenteral hyperalimentation. There have been many different methods of management. We report five premature infants with wounds of hyperalimentation fluid extravasation managed by the antibacterial ointment (Terramycin ophthalmic ointment™) and sesame oil and a antiinflammatory herbal mixture (MEBO™). The mean gestational age of patients was 31+2 weeks (range, 28+4 to 35+6 weeks), and the mean weight at extravasation was 1,930 g (range, 1,140 to 2,680 g). Extravasation occurred within the mean of 32 days (range, 17 to 50 days). The method of dressing was application of a thick layer of this mixture covered by vaseline and wet gauze renewed at an interval of 8-12 hr after irrigating the wounds thoroughly with normal saline. The mean duration of dressing was 30 days (range, 20-50 days). The wounds had healed completely leaving a small size of contracture without functional abnormality. We conclude that this therapy may be considered for an alternative treatment and warrants clinical trials for the confirmation of the local management of extravasation injury.
Newborn babies are particularly at risk for extravasation because of the fragility and small caliber of their peripheral veins. Moreover, they cannot localize pain allowing the infusion to continue unnoticed. If left untreated, these iatrogenic injuries can lead to extensive skin loss and damage to tendons, nerves and joints causing limb contractures (1, 2). In neonates, a conservative approach with or without delayed surgical repair is more appropriate (3). Many managements such as topical application of antiseptic cream (silver sulfadiazine with 0.2% chlorhexidine cream), enzymatic debridement, hyaluronidase application, subcutaneous saline flush-outs and liposuction have been used without randomized trials in neonates (4). In this study we managed the wound of the extravasation injury using the combination therapy of antibacterial ointment (Terramycin ophthalmic ointment™), sesame oil and anti-inflammatory herbal mixture (MEBO™) and systemic vitamin C.
In 1987, Xu (5) postulated that the conventional topical agents for burns were not good because they inhibited the ability of mesenchymal cells to differentiate and proliferate by exposing the injured cells prove to damage. As a result, he invented MEBO, which facilitates the drainage of necrotic tissue and mimics physiologic moist environment to provide an ideal condition for tissue to repair (4). Its main active component is β-sitosterol at a concentration of 0.25%. It is extracted from Philodendron amurense (Amur Cork tree). Other ingredients include Scutellariae Radix, Phellodendri Cortex, Coptidis Rhizoma, Astragali Radix for an anti-inflammatory effect and Scolopendrae Corpus for an antibacterial effect. This ointment comprises 17 amino acids, 14 fatty acids, 4 polysaccharides as well as various vitamins and trace elements in a base of beeswax and sesame oil. Clinical and experimental studies reported in the Chinese literature have demonstrated that β-sitosterol and beeswax facilitate wound healing (6-8). However, the moisturizing properties of MEBO can also cause wound infection due to the lack of antimicrobial capability. So the antibiotic ointment compensates the shortcomings of MEBO. In addition, instead of the usual method of MEBO with exposed dressing, we managed the wound with occlusive dressing with vaseline and wet gauze after irrigating thoroughly with normal saline.
We report on five premature infants with hyperalimentation fluid extravasation wounds managed by the combination therapy of antibacterial ointment, sesame oil and antiinflammatory herbal mixture (ASH mixture), and systemic vitamin C.
Patients included were preterm infants admitted from December 2003 to March 2004 in the neonatal intensive care unit of Ewha Womans University Mokdong Hospital. They were included after obtaining a signed informed consent from their parents. The procedures followed in the study were in accordance with the recognized ethical standards and with the Helsinki Declaration. The mean gestational age was 31+2 weeks (range 28+4 to 35+6 weeks), and the mean weight at extravasation was 1,930 g (range 1,140 to 2,680 g). Adverse events occurred within the mean of 32 days of birth (range, 17 to 50 days).
Patient 1 was born at 29+1 weeks gestation. Thirty six days after birth, subcutaneous swelling occurred on the dorsum of the right foot from extravasation of 12.5% total parenteral nutrition (TPN) and 10% intralipid (Fig. 1A). The wound was swollen with a bluish color change and scab formation. Dressing with an ASH mixture was started after removing the scab. After 4 weeks of dressing, the wound had healed completely only leaving pigmentation (Fig. 1B). On 126 days, full thickness skin necrosis that was measured at 1×1 cm2 developed after transfusion of pack red blood cells on the dorsum of the right hand (Fig. 2A). This was managed by the same dressing. After 5 weeks of dressing, the wound had healed with a small sized contracture without functional abnormality (Fig. 2C).
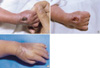
Patient 2, born at 33+1 weeks gestation, had subcutaneous swelling on the left forearm at 18 days after birth (Fig. 3). Intravenous 12.5% TPN had been administered via a peripheral cannula in the left forearm. Within 1 hr of swelling, the wound was managed by daily dressing with an ASH mixture. Over the next few days a partial thickness skin defect developed, which measured 0.5×1 cm2 in diameter. One month later the wound had healed completely.
Patient 3, born at 29+6 weeks, was found with swelling, a bluish color change, and a reddish blister on the dorsum of the left foot on 42 days after birth after extravasation of 12.5% TPN solution. A pale area on the tip of the left toe was apparent but showed good capillary return (Fig. 4A). As soon as the swelling was discovered, initial treatment was started with an ASH mixture with frequent saline irrigation and elevation of the left foot. A parenteral antibiotic (first generation cephalosporin) was started, and vitamin C given intravenously mixed in the fluid as an antioxidant was increased from 50-60 mg/day to 100-120 mg/day. Over the next 2 weeks, a full thickness skin ulcer with a whitish bed developed (Fig. 4B). Nineteen days after the lesion had been managed as mentioned, debridement and wet saline dressing was done. Twenty-four days after treatment, dressing was changed to potadine gauze dressing once or twice per day. The ulcer size decreased, and after 50 days of treatment the area had healed completely with a small size contracture without functional abnormalities (Fig. 4D).
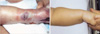
Patient 4, born at 28+4 weeks, had an extravasation injury from 12.5% TPN and 10% intralipid that had been infused into the lateral side of the right foot 50 days after birth (Fig. 5). This injury showed swelling, an erythematous color change, and a blister of the lateral side of the right foot. As soon as the swelling was found, dressing with an ASH mixture was started. After 20 days of treatment, the wound had healed completely with a small size of contracture. At 2 yr follow-up, small linear scar was revealed.
Patient 5 was born at 35+6 weeks. Seventeen days after birth, the lateral side of the left foot was affected with extravasation of 12.5% TPN and 10% intralipid. The wound showed swelling, a whitish skin color change, and a bullae measured at 2.5×1.5 cm2, but had a good capillary refill on the tip of the toe. As soon as the swelling was found, the foot was elevated and dressing with an ASH mixture was started. Parenteral vitamin C as an antioxidant was increased to a high dose. Twenty-six days after birth an eschar composing of necrosis and desquamation measuring at 1.5×1.5 cm2 developed (Fig. 6). After escharectomy, potadine gauze dressing was done once or twice per day and, oral antibiotics (second-generation cephalosporin) was administered. After 22 days of treatment, the wound had healed leaving a small size of contracture without functional abnormality. At 20 months of follow-up, the wound had healed completely without any sequale.
The method of dressing started with frequent and thorough irrigation of the wound with normal saline at the site of extravasation until the remainders were removed entirely for about 10 min. Then we applied a thick layer of the ASH mixture (2-3 mm) to the extravasated wound using a blunt instrument (tongue depressor) or cotton-tipped swabs, renewed at an interval of 8-12 hr with vaseline gauze covering. It was finally encircled by 4-5 layers of a wet gauze and was kept in an elevated position (9). We applied dressing at an interval of 8 hr at the acute stage and changing to an interval of 12 hr at the convalescent stage of wound.
The resultant wound quality was classified according to the wound complication and patients' satisfaction as excellent, good, fair, and poor. The absence of hypertrophic scar, contracture, and functional deformity with their parents' satisfaction was considered to be excellent. Poor had the existence of hypertrophic scar or contracture with functional deformity and dissatisfaction of their parents. Good and fair had an intermediate outcome between excellent and poor (Table 1).
Extravasation is the non-intentional leakage of infused fluid into the surrounding tissue, causing damage. Extravasation or infiltration of fluid has been known to occur in up to 70% of neonates, although tissue damage and skin necrosis are much less common (10). About 4% of infants leave the neonatal intensive care units with cosmetically or functionally significant scars, thought to be caused by extravasation injuries (11). Most injuries (70%) occurred in infants of 26 weeks of gestation and less. These very preterm infants have the most immature skin, which is easily damaged. They often require a longer duration of intravenous therapy, and obtaining intravenous access can be difficult (10).
In the literature, a number of treatments about managing these injuries are advocated, including hyaluronidase and saline infiltrations, hydrocolloids, and hydrogels (11). In this paper we report five cases with extravasation of parenteral hyperalimentation managed by the combination therapy of an ASH mixture and systemic vitamin C. As a result, no scar or functional abnormality was reported among the five cases. Although this study was limited by the rather small number of patients and by the lack of a control group for comparison, it is fair to conclude that the ASH mixture may be considered for an alternative treatment and warrants clinical trials for the confirmation of the local management of extravasation injuries without complications such as skin maceration, unmanageable excessive exudation, and wound infection.
There is no consensus on the management of extravasation injuries in preterm infants. Besides, no clinical trials have been performed that compare the outcome of management. Further research is needed to help prevent these injuries and to determine which is the best treatment to aid healing and reduce scarring. However, Zhang et al. (4) reported that the wound-healing rate by MEBO treatment is only better than the dry exposal therapy and did not differ from topical silver based antibacterial creams or even petroleum jelly. Moreover, Atiyeh et al. (12) reported that significant advantages were observed with the use of MEBO compared with that of Sofra-Tulle or Tegaderm semi-open dressing, including acceleration of the healing process. Thus it appears true that MEBO may provide a moist environment in favor of wound healing by preventing excessive water loss through the wounds. No adverse effects were reported with MEBO application in preterm infants. Retention of biologic fluids over the wound prevents desiccation of denuded dermis or deeper tissues and allows faster and unimpeded migration of keratinocytes over the wound surface. It also allows the naturally occurring cytokines and growth factors to exert their beneficial effect on wound contracture and re-epithelialization (12). Conversely moist environment also may provide moisture and nutrition necessary for bacterial proliferation. The infection may have cancelled out any beneficial effect by keeping the wound in a moist, physiologic environment (4). The major disadvantage of MEBO is the lack of antimicrobial capability, which may cause the infection and delay in healing the deep burn wound.
Therefore in this study we added an antibacterial ointment to MEBO. Ointments are soothing to apply, comfortable to wear, lubricating to the wound surface, and occlusive in nature. These mixtures deliver the antibiotic directly to the wound surface and limit scab formation (13). In spite of the usual method of the application of MEBO with exposed dressing, we managed the wound with occlusive dressing. Exposed dressing has two disadvantages. One is the complication of the rapid wiping-off of the substance owing to friction against clothes and bedding. The other is the exposure of physical trauma, foreign matter, and drying, which can induce the contamination of the wound. In addition, occlusive dressings promote the absorption of the wound exudates and the physical removal of surface necrotic tissue. However, it is difficult to keep the wound moist. Gauze probably does not provide a bacterial barrier, and if it is removed after drying on the wound, this may remove viable tissue and thus delay wound healing. So the gauze impregnated with Vaseline is applicated to this dressing. This can keep the wound in the moisturized environment (14, 15). After covering with Vaseline gauze, we enclosed the wound with 5-6 layers of wet gauze.
Once the injury has occurred, management falls into operative and non-operative categories. Non-operative treatment usually involves daily cleansing and topical application of an antibacterial ointment (16). Other investigators have reported experience with enzymatic debridement, hyaluronidase application, subcutaneous saline flush-outs, and liposuction (16-19). Surgical management of these injuries consists of debridement and skin grafting to the areas involved. Flap repairs of these injuries have also been reported successful (16-19). The decision to operate or maintain a conservative approach should be an ongoing process. Some authors advocate early intervention considering advantages that include the halting of an ongoing process and early wound closure (19). However, as the full extent of the injury may not declare itself for some time, multiple procedures may be required. There is also the possibility of removal of tissue that would have healed without intervention. In the patients of the present series, surgical debridement was delayed until the involved area was clearly demarcated. In cases 3 and 5, the preterm infants had the eschar on the dorsum and the lateral side of the foot after a few days of treatment, which was not initially present. We managed the wounds with the ASH mixture first, and then debridement the eschar. Healing is delayed with necrotic tissue in a wound. Eschar can create an environment that facilitates bacterial proliferation. Treatment with antibacterial ointment is frequently unsuccessful in eschar-covered wounds because the active ingredient is unable to penetrate the necrotic debris (13). So we managed our patients by debridement of the wound after eschar formation.
Ultimately it is better to prevent these injuries from occurring in the first place. If a substance known to cause an extravasation injury is to be used, it is important to ensure that the cannular is in an adequate vein and that this area is monitored regularly. Multiple puncture holes in the vein and obstructed venous systems should be avoided. And early intervention following extravasation can lessen the severity of tissue injury. The first steps after discovery of an infiltrated IV line are to discontinue the infusion and thoroughly examine the site. If the catheter appears to be lodged in the tissues, an attempt to aspirate any fluid remaining in the catheter can be made to lessen the amount of drug at the site (20). It remains paramount that all intravenous sites be watched and monitored very carefully (21).
Figures and Tables
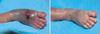 | Fig. 1(A) Extravasation with 12.5% total parenteral nutrition and 10% intralipid on the dorsum of the right foot. (B) Four weeks after treatment. |
 | Fig. 2(A) Extravasation with pack red blood cells on the dorsum of the right hand. (B) Eighteen days after treatment. (C) Five weeks after treatment. |
 | Fig. 3(A) Extravasation with 12.5% total parenteral nutrition on the left forearm. (B) Two months after treatment. |
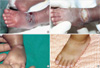 | Fig. 4(A) Extravasation with 12.5% total parenteral nutrition on the dorsum of left foot. (B) Eighteen days after treatment. (C) Fifty days after treatment. (D) Eighteen months after treatment. |
 | Fig. 5(A) Extravasation with 12.5% total parenteral nutrition and 10% intralipid on the lateral side of the right foot. (B) Two years after treatment. |
References
1. Yosowitz P, Ekland DA, Shaw RC, Parsons RW. Peripheral intravenous infiltration necrosis. Ann Surg. 1975. 182:553–556.

2. Brown AS, Hoelzer DJ, Piercy SA. Skin necrosis from extravasation of intravenous fluids in children. Plast Reconstr Surg. 1979. 64:145–150.

3. Kumar RJ, Pegg SP, Kimble RM. Management of extravasation injuries. ANZ J Surg. 2001. 71:285–289.

4. Zhang HQ, Yip TP, Hui I, Lai V, Wong A. Efficacy of moist exposed burn ointment on burns. J Burn Care Rehabil. 2005. 26:247–251.
5. Xu RX. Clinical uses of moist exposure therapy of burn. Zhong Xi Yi Jie He Za Zhi. 1988. 8:204–206.
6. Atiyeh BS, Ioannovich J, Magliacani G, Masellis M, Costagliola M, Dham R, Al-Musa KA. A new approach to local burn wound care: moist exposed therapy. A multiphase, multicenter study. J Burns Wound Care. 2003. 2:18–27.
7. Moon EJ, Lee YM, Lee OH, Lee MJ, Lee SK, Chung MH, Park YI, Sung CK, Choi JS, Kim KW. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 1999. 3:117–123.
8. Salatino A, Teixeira EW, Negri G, Message D. Origin and chemical variation of brazilian propolis. Evid Based Complement Alternat Med. 2005. 2:33–38.

9. Atiyeh BS, Ioannovich J, Magliacani G, Masellis M, Costagliola M, Dham R. Efficacy of moist exposed burn ointment in the management of cutaneous wounds and ulcers: a multicenter pilot study. Ann Plast Surg. 2002. 48:226–227.

10. Wilkins CE, Emmerson AJ. Extravasation injuries on regional neonatal units. Arch Dis Child Fetal Neonatal Ed. 2004. 89:274–275.

11. Cartildge PH, Fox PE, Rutter N. The scars of newborn intensive care. Early Hum Dev. 1990. 21:1–10.
12. Atiyeh BS, EI-Musa KA, Dham R. Scar quality and physiologic barrier function restoration after moist and moist-exposed dressings of partial-thickness wounds. Dermatol Surg. 2003. 29:14–20.

14. Ait-Aissa M. Advances in the use of SESAME OIL MIXTURE (a new approach in the method of application). Annal of Burns and Fire disasters. 2002. 15:1–4.
15. Atiyeh BS, Dham R, Costagliola M, Al-Amm CA, Belhaouari L. Moist exposed therapy: an effective and valid alternative to occlusive dressings for postlaser resurfacing wound care. Dermatol Surg. 2004. 30:18–25.

16. Upton J, Mulliken JB, Murray JE. Major intravenous extravasation injuries. Am J Surg. 1979. 137:497–506.

17. Lynch DJ, Key JC, White RR. Management and prevention of infiltration and extravasation injury. Surg Clin North Am. 1979. 59:939–949.

18. Gault DT. Extravastion injuries. Br J Plast Surg. 1993. 46:91–96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download