Abstract
To investigate if the surface modification of intraocular lens (IOL) is efficient in the prevention of posterior capsular opacification (PCO), the acrylic surface of intraocular lens (Acrysof®) was polymerized with polyethylene glycol (PEG-IOL). The human lens epithelial cells (1×104 cells/mL) were inoculated on PEG grafted or unmodified acrylic lenses for the control. The adherent cells on each IOL surface were trypsinized and counted. The every PEG-IOL was implanted in 20 New Zealand rabbits after removal of crystalline lens. The formations of PCO were checked serially through retroilluminated digital photography, and the severity scores were calculated using POCOman®. The cell adherence patterns on each IOL were examined by scanning electron microscopy. As a result, the mean number of adherent cells of PEG-IOL (3.2±1.1×103) tended to be smaller than that of the acrylic controls (3.6±1.9×103) without a statistical significance (p=0.73). However, the mean severity of PCO formation in PEG-IOL was significantly lower than that in the control during the third to sixth weeks after surgery. Scanning electron microscopy revealed that the more patch-like cells were found firmly attached to the IOL surface in control than in the PEG-IOL. Conclusively, PEG polymerization to the acrylic IOL would possibly lessen the formation of PCO after cataract removal.
Despite the rapid progress made in modern cataract surgery, posterior capsular opacification (PCO) still remains as one of the most common postoperative complications. Usually the opacification is minimal and confined to the periphery and is not of great concern. However, in some severe cases, it impairs vision and can make previous cataract removal worthless. Thus, additional intervention is often necessary for visual recovery. The most well known method to overcome PCO is neodymium:YAG capsulotomy, which is straightforward and immediately effective, but perhaps the best way of dealing with PCO is to prevent its occurrence. As PCO is thought to be formed by the migration and metaplasia of lens epithelial cells (LECs), it may be beneficial to remove intraoperatively as much of the cortical remnant and LEC nests as possible, though admittedly there is some dispute in this regard (1-3).
Many theories have been put forward to preclude the formation of posterior capsular opacities, and attempts to modify interactions between lens capsules and intraocular lens (IOL) surfaces are abundant. Recently lens design, especially the configuration of the optic edge have been found to be most effective at preventing PCO, as the edge itself could act as a mechanical barrier against LEC migration and could induce capsular bending (4-6). Many attempts have also been made to identify an ideal lens material and gradually it has been accepted that hydrophobic acrylic plastic offers the best means of reducing PCO and postoperative inflammation (7). Moreover, the lens surface may also play a primary role in LEC adhesion, and thus, development programs have been initiated to produce less adhesive surfaces. However, few results are available due to the tardy progress made in the synthesis of biocompatible materials (8-11). In this study, we investigated the effect of surface modification with polyethylene glycol (PEG); a type of hydrophilic polymer that is known to be effective at reducing protein deposition and cell adhesion. In our previous studies, graft polymerization of PEG to polymethylene methacrylate (PMMA) was found to be effective at reducing keratocyte adhesion, and therefore, we further investigated the efficacy of a PEG graft on an acrylic lens with a square edge, which is presumed to be the best design and materials in terms of reducing PCO (12).
Acrylic lenses with a square edge of 6 mm optic size (SA 60AT®, Alcon) were used, and graft polymerization was done as described in our previous study (12). Briefly, the lens surface was first treated with oxygen plasma, which was generated under low pressure (70 torr) at 100 Watt, and then it was incubated in PEG solution at 60℃ for 24 hr.
IOLs were sterilized with ethylene oxide gas and washed with phosphate buffered saline (PBS) before use. The two haptics were severed and the remaining optic portions were placed at each bottom of 96-well culture plate. As many as 1×104 immortalized human lens epithelial cells (B-3, ATCC, U.S.A.) were layered onto the optics and incubated for 24 hr at 37℃ in 5% CO2. The medium was then removed and IOL surfaces were washed twice carefully with PBS to remove non-adherent cells. The 0.25% trpysin (200 µL) was then added and incubated for 10 min at 37℃ to harvest adherent cells. Cells were then placed in the mixture of 400 µL of PBS and 400 µL of minimal essential medium (MEM) containing 20% fetal bovine serum, and finally centrifuged at 1,500 rpm for 5 min. Pellets were resuspended in MEM (200 µL), stained with trypan blue and counted in a hemocytometer (Superior/Marienfeld, D-97965, Bad Mergentheim, Germany). A total of nine samples were tested per group. To verify statistical significances, we used the Mann-Whitney U test.
In accord with the principles of ARVO Statements on the Use of Animals in Ophthalmic and Vision Research, 20 PEG grafted acrylic lenses (PEG-IOL) and 20 control uncoated lenses (SA 60AT®) were implanted randomly into either eye of male New Zealand white rabbits weighing from 2.5-3.0 kg. Briefly, animals were anesthetized with an intramuscular injection of ketamine hydrochloride (100 mg/kg) (Yuhan, Seoul, Korea) and xylazine (5 mg/kg) (Rompun®, Bayer, Leverkusen, Gemany). Pupils were dilated with tropicamide (Mydriacyl®, Alcon, Fortworth, U.S.A.) 3 times at 10-min intervals prior to surgery. Operations were conducted under sterile condition using a Zeiss surgical microscope by one surgeon. The anterior chamber was entered through a 2.75 mm sized superior self sealing clear corneal incision, and side entry was made 90 degrees apart from the initial incision using a 15° standard angle blade. Using a visosurgical device (Viscoat®, Alcon), a 4.5 to 5.0 mm round, well centered continuous curvilinear capsulotomy was made with a capsulorhexis forceps, such that the anterior capsular margin covered the whole optic margin completely. A complete hydrodissection followed by hydrodelineation was performed and the remaining lens material was removed by phacoemulsification (Premier®, Storz, Germany). The capsular bag was fully inflated with viscoelastics, and a random IOL was inserted using an injector (Monarch®, Alcon, Korea). Then IOL was positioned in the desired central position with 360 degree anterior capsular coverage. After removal of the remaining whole viscoelastics, the wound was closed with one 10-0 monofilament nylon suture in all cases. Because the rabbit cornea is relatively thin, it was sometimes difficult to make a complete self sealing clear corneal incision. Postoperatively a mixture of dexamethasone, neomycin, and polymixin B sulfate ointment (Maxitrol®, Alcon, Fortworth, U.S.A.) was applied 3 times daily for 7 days. Complications were evaluated at weekly and standardized retro-illuminated photographs were taken through a dilated pupil until the 8th postoperative week by the same examiner using a light source with the same size and angle. To nullify the effects of the anterior capsule in the formation of PCO, we excluded those rabbits with implanted lens optic margins not covered completely by the anterior capsule or extruding into the anterior chamber. The severity of PCO was graded using semi-objective calculation software (POCOman®) from St. Thomas's Hospital and the King's College London imaging process group (Fig. 1). Results were compared using the nonparametric rank summation technique and the Mann-Whitney U test, and significance was accepted at the 5% confidence level. At the end of our study, 8 weeks after implantation, all animals were sacrificed and implanted IOLs were carefully removed. The IOLs were again fixed in glutaraldehyde (2.5%), washed with 0.1 M/L PBS, dehydrated in graded ethanol, critical-point-dried in liquid CO2 (CPD 7501), and coated with gold (Giko-IB-3) for scanning electron microscopy (JSM-300, Joel, Tokyo, Japan). Adherent cells were counted in 5 or more fields at 200× and statistical significances between study groups were determined using the Student's t-test.
The mean number of adherent LEC's harvested from each lens surface was 3.6±1.9×103 for the control and 3.2±1.1×103 for the PEG-IOL, which was not significant (p=0.73, Fig. 2).
No intraoperative complications developed, e.g., capsular tear. However, during follow-up, some parts of IOLs were found to have extruded outside the capsular bag in 7 (4 in the control, 3 in the PEG-IOL group), and thus complete anterior capsular envelopment over the optic was impossible. These rabbits were excluded from the study. Minimal anterior chamber reactions and mild corneal edema were resolved during the first week. The formation of PCO was evident from the third week after surgery and grew weekly until the last follow up. In the PEG-IOL group, PCO began to take on a fine fibrotic appearance rather than a conglomerated cellular proliferation like the Elschnig's pearl in the control group, and became a similar figure later on (Fig. 3A, B). The PCO formation was significantly lower during the third to sixth weeks in the PEG-IOL group (Table 1, Fig. 3C, Mann-Whitney-U test). However, during the final two weeks (weeks 7 and 8), no significant differences were found, and some rabbits showed marked LEC proliferation starting from the optic-haptic junction, regardless of group (Fig. 4). Scanning electron microscopic examinations of IOL surfaces revealed fewer adherent LECs on PEG-IOLs. Mean numbers were 26.4±13.7 for the control and 16.2±11.2 for the PEG -IOL, which were significantly different (p=0.041, Mann-Whitney-U test). Regarding the appearance, more cells were found to be firmly attached to control lens surfaces with a patch-like figure (Fig. 5).
The formation of PCO is believed to be a multifactorial process. The surgical technique, lens material, and configuration including optic edge design and haptic angulations are thought to play important roles in PCO formation, though patient condition and the postoperative administration of drugs may also have an influence (4, 6, 13, 14). However, there are many different opinions concerning the pathogenesis of PCO (2, 3). In general, the optic material is not regarded so important as optic configuration in the pathogenesis of PCO, although the adhesion of various inflammatory cells and fibronectin is clearly optic material and surface-dependent (15, 16). Thus, surface modifications, including heparin incorporation, have been examined, and some reports have shown favorable results under selective conditions (8-11). We undertook this study to investigate the effect of surface modifying acrylic lenses with PEG, which is a high molecular weight hydrophilic polymer and is tethered on the surface, and thus the remainder of the molecule is free to move and can prevent the deposition of protein materials and inflammatory cells (17-19). In spite of some debates, hydrophobic acrylic lenses are known to have an affinity for fibronectin, and thus, we consider that PEG grafting might have a beneficial effect (20). Moreover, although acrylic lenses are better at preventing PCO than other type of IOL, PCO formation can also be influenced profoundly by other physical characteristics of IOL, and therefore, in the present study we compared the effect of PEG surface grafting on optic materials of same design (7).
In our previous study, PEG-grafted PMMA had a markedly lower contact angle and reduced cellular adhesion (12). However, in the present study, we found no significant differences between the two study groups in vitro, though a tendency for cells to adhere less was observed for the PEG-acrylate. We consider this is due to the characteristics of materials that compose the IOL. Since acrylates are soft and their hardness can change markedly with the sudden drop in temperature during washing, their adhesiveness might also be changed accordingly.
When PCO formations were compared, it was found to be significantly less severe in the PEG-IOL group than the in control group from the third week postoperatively, when sufficient amount of time passed for the cells to migrate and proliferate themselves. Moreover, the opacification appeared as a fine fibrosis in the PEG-IOL group, suggesting reduced visual impairment for the PEG-IOL. However, this PCO lowering effect did not last for long, showing no differences from seventh week of examination. And some rabbits in both groups revealed enormous LEC proliferation starting from the optic-haptic junction, and soon covering throughout the optic surface. The acrylic lens used in our study is a single piece and considered to be better at stretching the posterior capsule because it exerts even forces on the posterior capsule after insertion into the capsular bag, and at the same time avoids early posterior capsular striae, which is believed to be the route of early LEC migration (21, 22). However, the junction between optic and haptic is large and there is no definite barrier, and thus, it can act as a gate for LEC migration. In this study, LECs entered the junction at around 1 month and spread very rapidly to the whole optic surface (Fig. 3). The amount and rapidity of this migration were so great that after six weeks our results might have lost statistical significance. This demonstrates that such modifications of IOL surface could work during the early phase of PCO formation and that the lens configuration might have a greater effect after a certain time period, as PCO formation is a multifactorial process.
The limitation of our study is that it involved a relatively small number, and thus, a further study using a larger sample number is needed to confirm the findings of this study. Another limitation is that as we used only one type of IOL, our results cannot be generalized to other lenses with different contours. So it is also needed to study various lens materials and configurations to elucidate their efficacies in terms of preventing PCO formation. Although the formation of PCO is multifactorial and it seems to be impossible to consider its all causative factors in one study, the inflammation induced by the surgery is sure to have significant influence. To investigate its role, more frequent examinations in early postoperative phases are also needed.
In conclusion, the surface modification of acrylic IOL with PEG may reduce the formation of PCO during the early stage. However, the multifactorial nature of PCO means that we should always consider that other contributors are likely to be important.
Figures and Tables
Fig. 1
The severity grades of posterior capsular opacification with the POCOman software. A retroilluminated picture (A) is transformed into a black and white picture (B). The margin of the anterior capsule should be marked then the grid is allocated automatically. Using the standard photographies (grade 1-3), the severity score is calculated with the formula as follows: severity score=(3*Red [grade3]+2*Yellow [grade2]+1*Blue [grade1]/total area).
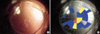
Fig. 2
In vitro human lens epithelial cell adhesion after 24-hr culture. The averaged number was slightly higher in the control than that of PEG grafted lens without statistical significance. p=0.73 by Mann whitney-U test.
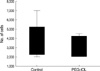
Fig. 3
The formation of posterior capsular opacification (PCO) after implantation of intraocular lens in rabbits. (A) Polyethylene glycol (PEG), (B) Control. At the 3rd week, the fine fibrotic materials were found on the posterior capsule with minimal proliferation of lens epithelial cells (LECs) in comparison with the control, where the conglomerated LEC's were dominant. (C) The severity of PCO was significantly lower from the third week in the PEG group until the sixth week of examination. *, p<0.05 by Mann Whitney-U test.
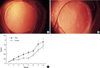
Fig. 4
An enormous proliferation of lens epithelial cells through the optic-haptic junction. (A) 4th week. Few cells were seen except small cell buds at the optic-haptic junction (arrowhead). (B) 6th week. The cell nests were rapidly growing and threatening the visual axis. (C) 8th week. The visual axis was completely obliterated by enormously proliferating cells.
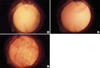
Fig. 5
The scanning electron microscope of adherent cells (×200 fold). (A) In the control group, spindle shape, patch-like cells, together with a few small round cells, were firmly attached to lens surface. (B) In the polyethylence glycol (PEG) group similar cells were found but seemed less adherent.

References
1. Peng Q, Apple DJ, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, Schoderbek R, Guindi A. Surgical prevention of posterior capsule opacification. Part 2: enhancement of cortical cleanup by focusing on hydrodissection. J Cataract Refract Surg. 2000. 26:188–197.
2. Linnola RJ, Werner L, Pandey SK, Escobar-Gomez M, Znoiko SL, Apple DJ. Adhesion of fibronectin, vitronectin, laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. Part 2: explanted intraocular lenses. J Cataract Refract Surg. 2000. 26:1807–1818.
3. Johnston RL, Spalton DJ, Hussain A, Marshall J. In vitro protein adsorption to 2 intraocular lens materials. J Cataract Refract Surg. 1999. 25:1109–1115.

4. Nishi O, Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg. 1999. 25:521–526.

5. Nishi O, Nishi K, Menapace R. Capsule-bending ring for the prevention of capsular opacification: a preliminary report. Ophthalmic Surg Lasers. 1998. 29:749–753.

6. Nishi O, Nishi K, Sakanishi K. Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthalmic Surg Lasers. 1998. 29:587–594.

7. Tanaka T, Yamakawa N, Mizusawa T, Usui M. Interaction between inflammatory cells and heparin-surface-modified intraocular lens. J Cataract Refract Surg. 2000. 26:1409–1412.

8. Trocme SD, Li H. Heparin-Surface-Modified Lens Study Group. Effect of heparin-surface-modified intraocular lenses on postoperative inflammation after phacoemulsification: a randomized trial in a United States patient population. Ophthalmology. 2000. 107:1031–1037.
9. Yuan Z, Sun H, Yuan J. A 1-year study on carbon, titanium surface-modified intraocular lens in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2004. 242:1008–1013.

10. Lundvall A, Zetterstrom C, Lundgren B, Kugelberg U. Effect of 3-piece AcrySof and downsized heparin-surface-modified poly (methyl-methacrylate) intraocular lenses in infant rabbit eyes. J Cataract Refract Surg. 2003. 29:159–163.
11. Gatinel D, Lebrun T, Le Toumelin P, Chaine G. Aqueous flare induced by heparin-surface-modified poly(methyl methacrylate) and acrylic lenses implanted through the same-size incision in patients with diabetes. J Cataract Refract Surg. 2001. 27:855–860.

12. Kim MK, Park IS, Park HD, Wee WR, Lee JH, Park KD, Kim SH, Kim YH. Effect of poly (ethylene glycol) graft polymerization of poly (methyl methacrylate) on cell adhesion. In vitro and in vivo study. J Cataract Refract Surg. 2001. 27:766–774.
13. Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trial. Am J Ophthalmol. 1999. 128:271–279.

14. Fernandez V, Fragoso MA, Billotte C, Lamar P, Orozco MA, Dubovy S, Willcox M, Parel JM. Efficacy of various drugs in the prevention of posterior capsule opacification: experimental study of rabbit eyes. J Cataract Refract Surg. 2004. 30:2598–2605.

15. Smith SR, Daynes T, Hinckley M, Wallin TR, Olson RJ. The effect of lens edge design versus anterior capsule overlap on posterior capsule opacification. Am J Ophthalmol. 2004. 138:521–526.

16. Nishi O, Nishi K, Osakabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg. 2004. 30:2170–2176.
17. Desai NP, Hubbell JA. Biological responses to polyethylene oxide modified polyethylene terephthalate surfaces. J Biomed Mater Res. 1991. 25:829–843.

18. Lee JH, Kopecek J, Andrade JD. Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J Biomed Mater Res. 1989. 23:351–368.

19. Han DK, Park KD, Ryu GH, Kim UY, Min BG, Kim YH. Plasma protein adsorption to sulfonated poly (ethylene oxide)-grafted polyurethane surface. J Biomed Mater Res. 1996. 30:23–30.
20. Kim CK, Lee JA, Tchah HW. Adhesion and morphologic change of lens epithelial cell according to materials of intraocular lens. J Korean Ophthalmol Soc. 2003. 44:445–453.
21. Lane SS, Burgi P, Milios GS, Orchowski MW, Vaughan M, Schwarte E. Comparison of the biomechanical behavior of foldable intraocular lenses. J Cataract Refract Surg. 2004. 30:2397–2402.

22. Kim TW, Kwon JW, Lee JH. Long-term follow-up of the insertion of capsular tension ring in cataract surgery. J Korean Ophthalmol Soc. 2005. 46:1624–1629.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download