Abstract
Long-segment tracheal stenosis in infants and small children is difficult to manage and can be life-threatening. A retrospective review of 12 patients who underwent surgery for congenital tracheal stenosis between 1996 and 2004 was conducted. The patients' median age was 3.6 months. All patients had diffuse tracheal stenosis involving 40-61% (median, 50%) of the length of the trachea, which was suspected to be associated with complete tracheal ring. Five patients had proximal bronchial stenosis also. Ten patients had associated cardiac anomalies. Three different techniques were performed; pericardial patch tracheoplasty (n=4), tracheal autograft tracheoplasty (n=6), and slide tracheoplasty (n=2). After pericardial tracheoplasty, there were 2 early and 2 late deaths. All patients survived after autograft and slide tracheoplasty except one who died of pneumonia one year after the autograft tracheoplasty. The duration of ventilator support was 6-40 days after autograft and 6-7 days after slide tracheoplasty. The duration of hospital stay was 13-266 days after autograft and 19-21 days after slide tracheoplasty. Repeated bronchoscopic examinations were required after pericardial and autograft tracheoplasty. These data demonstrate that pericardial patch tracheoplasty show poor results, whereas autograft or slide tracheoplasty gives excellent short- and long-term results.
Long-segment tracheal stenosis in infants and children is difficult to manage and can be life-threatening (1). Patients usually have associated cardiac and other anomalies. The rare incidence of congenital tracheal stenosis makes it difficult to develop a standard management protocol. Several surgical techniques have been introduced, including rib cartilage tracheoplasty (2), pericardial patch anterior tracheoplasty (3), resection and end-to-end anastomosis (4), slide tracheoplasty (5), tracheal homograft (6), and free tracheal autograft (7). Here, we review our experience performing three different techniques (pericardial patch anterior tracheoplasty, tracheal autograft tracheoplasty, and slide tracheoplasty) in 12 patients.
Between 1996 and 2004, 12 consecutive patients underwent surgery for congenital tracheal stenosis at Samsung Seoul Hospital. All patients showed life-threatening respiratory distress, except one patient who visited the outpatient clinic because of recurrent respiratory infection. Their age at surgery was 13 days-2.8 yr (median, 3.6 months), and body weight was 2.9-13.6 kg (median, 4.8 kg). Eight patients were boys and four were girls. Eight patients were transferred while intubated; the duration of intubation before surgery was 3-57 days (median, 10 days). In five patients, tracheal stenosis was detected after other surgical interventions. All patients were evaluated with cardiac echocardiography, and computed tomography (CT) scans or bronchoscopy. All patients had diffuse tracheal stenosis involving 40-61% (median, 50%) of the length of the trachea, which was suspected to be associated with complete tracheal ring. Five patients also had proximal bronchial stenosis. Seven patients had a pulmonary artery sling. One patient had a tracheal origin of the right upper lobe bronchus, which was obstructed in association with lobar agenesis. These patients' characteristics are summarized in Tables 1 and 2. Operative indications were the presence of severe respiratory symptom and congenital tracheal stenosis associated with complete tracheal ring or pulmonary artery sling. No patient with the same diagnosis was treated conservatively during the same period.
The surgical approach was done through a median sternotomy. In two patients, this procedure was the second time of entry to the thorax by a sternotomy. Cardiopulmonary bypass was used in all patients to provide safe respiratory support during the tracheal repair. Bicaval cannulation was used in patients requiring the repair of intracardiac defects, and single right atrial cannulation was used for the others. Intracardiac procedures were performed first, followed by the repair of the pulmonary artery sling, and by tracheal repair. Intracardiac procedures were performed simultaneously in three patients. Correction of the pulmonary artery sling was performed simultaneously in seven patients (Table 2). In six of these seven patients, the left pulmonary artery (LPA) was transected at its origin, moved anterior to the trachea, and reimplanted to the main pulmonary artery. In one patient, the LPA was translocated anterior to the trachea after dividing the trachea, as described by Jonas et al. (4) For tracheal repair, we used three different techniques - pericardial patch tracheoplasty in four patients, autograft tracheoplasty in six patients, and slide tracheoplasty in two patients.
Pericardial patch tracheoplasty was our procedure of choice between 1995 and 1998. After a median sternotomy, a piece of pericardium was harvested and fixed in 0.625% glutaraldehyde solution. The anterior surface of the trachea was dissected and incised through the extent of the complete tracheal rings. The pericardial patch was sutured in place using interrupted 6-0 polypropylene sutures, starting from the most inferior part of the incision adjacent to the carina. Several sutures were placed on the pericardial patch and surrounding mediastinal tissues to suspend the patch. The endotracheal tube was positioned so that the tip should be placed just above the carina. Hemoclips were placed at the superior and inferior ends of the patch. The mediastinum was filled with saline, and the patient was ventilated with a peak airway pressure up to 40 cmH2O to assess the presence of any leaks around the patch. The suture line was sealed with fibrin glue. The patients were paralyzed and ventilated for one week.
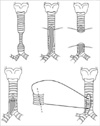
Tracheal autograft tracheoplasty has been our procedure of choice since 1998. After extensive mobilization of the trachea from the cricoid cartilage to the hilum, the anterior trachea was incised through the extent of the complete tracheal ring. The tracheal autograft was harvested at the midportion of the opened trachea after assessing how much of the trachea could be resected. The remaining ends of the trachea were anastomosed posteriorly with multiple interrupted 7-0 polydioxanone (PDS II, Ethicon, Inc., Edinburgh, U.K.) sutures. To avoid exposing the threads in the lumen of the trachea, the sutures were placed not to penetrate the whole layer of the trachea and were tied externally to keep the knots out of the tracheal lumen. After anastomosis of the posterior wall, the autograft segment was trimmed and patched to cover the anterior defect. In patients with an associated main bronchial stenosis, the anterior incision of the trachea was extended to the stenotic bronchial origin. We divided the autograft segment into two pieces and covered the bronchial defect sepa-rately (Fig. 1) to avoid the use of the pericardium. Hemoclips were placed to identify this part of the autograft. In one patient, the autograft was not long enough to cover the anterior opening of the trachea and bronchus, the pericardial patch was used additionally.
We have recently used slide tracheoplasty in two patients. After mobilization, the trachea was transected at the midportion of the stenotic trachea. The superior trachea was incised posteriorly, the inferior trachea was incised anteriorly through the entire stenotic portion, and the corners of both ends of the resected trachea were trimmed. The two components were slid together and anastomosed with multiple interrupted sutures using 7-0 polydioxanone sutures. The endotracheal tube was positioned in the middle of the trachea.
There were four operative deaths (33%) and one late death; all operative deaths occurred after pericardial patch tracheoplasty and eventually, no patient in the pericardial patch group survived. A 13-day-old boy (patient No. 1) with a history of central shunt due to pulmonary atresia with ventricular septal defect (VSD) underwent pericardial patch tracheoplasty. Postoperative bronchoscopic examination revealed distal tracheomalacia but no granulation tissue or stenosis. The patient had a difficulty in weaning from the ventilator and died of sepsis on postoperative day 29. Patient No. 2, a 5-month-old boy, underwent tracheoplasty and LPA reimplantation to treat a pulmonary artery sling. The patient required prolonged ventilator support because of respiratory syncytial virus infection (verified by culture). Repeated bronchoscopy revealed diffuse distal tracheal stenosis and stenosis in both bronchi. We could not treat the recurrent stenosis any further, and the patient died of respiratory failure on postoperative day 110. Patient No. 3, a 1.6-month-old girl, also underwent pericardial patch tracheoplasty and LPA reimplantation. Although postoperative bronchoscopy showed no granulation tissue or stenosis, high fever and pneumomediastinum developed after the bronchoscopy. Re-exploration was required and revealed tracheal disruption. The patient died of sepsis and respiratory failure on postoperative day 31. Patient No. 4 had a history of one-stage repair of coarctation of the aorta and VSD, could not be weaned from the ventilator and showed diffuse congenital tracheal stenosis. He underwent pericardial patch tracheoplasty extending to the right main bronchus. After the initial tracheoplasty, he suffered from diffuse distal tracheal stenosis. Surgery was performed again using rib cartilage, but he died of an accidental tension pneumothorax during the tracheostomy one month later.
No early deaths occurred in patients treated with autograft tracheoplasty and slide tracheoplasty. No complications were associated with cardiopulmonary bypass. The duration of ventilator support was 6-40 days after tracheal autograft and 6-7 days after slide tracheoplasty. The duration of hospital stay was 13-266 days after tracheal autograft and 19-21 days after slide tracheoplasty. The need for repeated bronchoscopy was less in the patients treated with slide tracheoplasty (Table 3). One late death (13%) occurred in a patient treated with an autograft. Patient No. 10 underwent the Rastelli operation using a porcine valved conduit for TOF with single coronary artery, and autograft tracheoplasty. The patient experienced respiratory distress syndrome postoperatively and should have had prolonged ventilator support. Bronchoscopy revealed diffuse residual tracheal stenosis and multiple granulomas. He underwent bronchoscopic examinations repeatedly (10 times) and dilatation with bougienage. During the same period of hospitalization, the prosthetic valved conduit had to be changed to a homograft because of fungal endocarditis. He was discharged on postoperative day 266, but died of sudden respiratory failure associated with pneumonia one year after surgery.
The median follow-up period for the survivors was 2.6 yr (9 months to 5.7 yr). All surviving patients are currently asymptomatic (Table 4).
Congenital tracheal stenosis associated with complete tracheal rings presents with various symptoms according to the grade of stenosis. Long-segment tracheal stenosis often presents as severe respiratory distress within a few weeks after birth and is associated with other cardiac anomalies, pulmonary artery sling, and abnormalities of the digestive system. It should be considered an airway problem when the patient presents with predominantly respiratory symptoms or shows an unusual perioperative course after cardiac surgery (8). Long-segment tracheal stenosis is sometimes very difficult to diagnose and manage adequately (1). A recent study of multicenter analysis revealed that the overall mortality is still high (28%) after surgical intervention and the highest mortality rate was observed in patients younger than 1 month (73%) and in those with intracardiac anomalies (53%) (9). Because of the rarity of congenital tracheal stenosis and the difficulty of treatment, several surgical techniques have been proposed without a standard protocol.
At our institution, we first used the anterior pericardial tracheoplasty technique in four patients, with adverse results. The theoretical advantages of using pericardium are that the pericardium is easily accessible, a generous amount can be harvested without compromising other structures, and the pericardium molds easily and can be tailored to fit specific locations (10). There are several advantage of anterior tracheoplasty: 1) no restriction on the patient's size, 2) no technical limitations as to the length and the location of the stenosis, 3) a good blood supply by preserving the lateral wall of the trachea, 4) avoiding damage to the recurrent laryngeal nerve, and 5) the possibility of concomitant repair of other cardiac anomalies (3, 10-12). However, this technique also has problems, such as granulation tissue, restenosis, patch tracheomalacia, and the prolonged hospital stay (10-13). The major disadvantage is excessive granulation formation on the mesenchymal surface of the tracheal substitute, needing frequent postoperative bronchoscopy (11, 14). The largest study of clinical experience with the pericardial patch tracheoplasty was reported by the group at the Children's Hospital in Chicago (13). Although their operative mortality rate was low (7%), their patients exhibited that the mean hospital stay was 60 days, a significant late mortality rate (18%) and a high reoperation rate (25%) for residual stenosis.
Although this technique was applied early in our series, the results were disappointing. All patients treated with this method could not be discharged from the hospital. Two of these patients showed restenosis as described above. Repeated bronchoscopic intervention because of the growth of granulation tissue can cause problems, as shown in our patient who experienced rupture of the trachea and infection. A long hospital stay also can be a problem.
Backer et al. were the first to describe the free tracheal autograft tracheoplasty. They also reported good results in 15 patients with 1 surgical mortality (7%) and 1 late death (7%) (13). The advantages of the autograft technique include 1) the use of all autologous materials for the repair, 2) the technical ease of using only anterior sutures, 3) the already-present respiratory epithelial lining of the autograft, 4) intrinsic maintenance of the cartilage contour, 5) potential for growth, and 6) ready availability because the trachea in these infants is often excessively long (13, 15, 16). We have used autograft techniques in six patients without operative mortality. One patient had a composite graft of a tracheal autograft and the pericardium. He had postoperative granulation tissue and mild stenosis at the part of pericardial graft and underwent repeated bronchoscopy to remove the granulation tissues. We used the free tracheal autograft, which was divided into two pieces, to repair the orifice of the stenotic main bronchus and to avoid using the pericardium in four patients (Fig. 1). Tracheal stenosis is frequently associated with stenosis of the main bronchi, as seen in our data. There are two options in this situation: the first is to leave the stenosis of the main bronchi and the second is to correct the stenosis simultaneously. We tried to correct the stenosis of the main bronchi with the segment of the tracheal autograft with good results.
The slide tracheoplasty technique was first reported in two patients by Tsang et al. in 1989 (5) and modified by Grillo et al. (17, 18). The advantages of this technique include reconstructing the trachea with tracheal tissue, which provides a stable cartilaginous wall with normal epithelial lining; preserving the lateral blood supply, not imparing tracheal growth (19) and avoiding cardiopulmonary bypass (18). However, in a small number of patients, it is safe and appropriate to perform the procedure under cardiopulmonary bypass. In our series, we performed slide tracheoplasty under cardiopulmonary bypass with good results. Although recently reported mortality rates were various (0-25%), slide tracheoplasty is preferred for congenital tracheal stenosis by many authors because of uneventful postoperative course (9, 14, 20, 21). Recently, we do not perform bronchoscopy as a routine procedure because CT scan with 3-dimensional reconstruction is less invasive and we consider the resolution and accuracy is reliable (22).
Long-segment congenital tracheal stenosis is a life-threatening problem that is frequently associated with a pulmonary artery sling or intracardiac defects. It should be considered an airway problem when the patient presents with predominantly respiratory symptoms or shows an unusual perioperative course after cardiac surgery. Selection of the surgical strategy to treat long-segment congenital tracheal stenosis depends on the anatomical pattern of the stenosis and should be individualized. Slide tracheoplasty is our recent procedure of choice. However, we prefer the free tracheal autograft tracheoplasty in case of concomitant bronchial stenosis. We believe that the close cooperation between pediatric pulmonologists, cardiologists, radiologists, pediatric cardiac surgeons, and pediatric otolaryngologists is essential for optimal results.
Figures and Tables
Fig. 1
Tracheoplasty using divided two pieces of tracheal autograft in a patient with combined stenosis of bronchial origin.
References
1. Benjamin B, Pitkin J, Cohen D. Congenital tracheal stenosis. Ann Otol Rhinol Laryngol. 1981. 90:364–371.

2. Kimura K, Mukohara N, Tsugawa C, Matsumoto Y, Sugimura C, Murata H, Itoh H. Tracheoplasty for congenital stenosis of the entire trachea. J Pediatr Surg. 1982. 17:869–871.

3. Idriss FS, DeLeon SY, Ilbawi MN, Gerson CR, Tucker GF, Holinger L. Tracheoplasty with pericardial patch for extensive tracheal stenosis in infants and children. J Thorac Cardiovasc Surg. 1984. 88:527–536.

4. Jonas RA, Spevak PJ, McGill T, Castaneda AR. Pulmonary artery sling: primary repair by tracheal resection in infancy. J Thorac Cardiovasc Surg. 1989. 97:548–550.

5. Tsang V, Murday A, Gillbe C, Goldstraw P. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg. 1989. 48:632–635.

6. Elliott MJ, Haw MP, Jacobs JP, Bailey CM, Evans JN, Herberhold C. Tracheal reconstruction in children using cadaveric homograft trachea. Eur J Cardiothorac Surg. 1996. 10:707–712.

7. Backer CL, Mavroudis C, Dunham ME, Holinger LD. Repair of congenital tracheal stenosis with a free tracheal autograft. J Thorac Cardiovasc Surg. 1998. 115:869–874.

8. Pfammatter JP, Casaulta C, Pavlovic M, Berdat PA, Frey U, Carrel T. Important excess morbidity due to upper airway anomalies in the perioperative course in infant cardiac surgery. Ann Thorac Surg. 2006. 81:1008–1012.

9. Chiu PP, Kim PC. Prognostic factors in the surgical treatment of congenital tracheal stenosis: a multicenter analysis of the literature. J Pediatr Surg. 2006. 41:221–225. discussion -5.
10. Cosentino CM, Backer CL, Idriss FS, Holinger LD, Gerson CR, Mavroudis C. Pericardial patch tracheoplasty for severe tracheal stenosis in children: intermediate results. J Pediatr Surg. 1991. 26:879–884. discussion 85.

11. Bando K, Turrentine MW, Sun K, Sharp TG, Matt B, Karmazyn B, Heifetz SA, Stevens J, Kesler KA, Brown JW. Anterior pericardial tracheoplasty for congenital tracheal stenosis: intermediate to long-term outcomes. Ann Thorac Surg. 1996. 62:981–989.

12. Heimansohn DA, Kesler KA, Turrentine MW, Mahomed Y, Means L, Matt B, Weisberger E, Brown JW. Anterior pericardial tracheoplasty for congenital tracheal stenosis. J Thorac Cardiovasc Surg. 1991. 102:710–715. discussion 5-6.

13. Backer CL, Mavroudis C, Holinger LD. Repair of congenital tracheal stenosis. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002. 5:173–186.
14. Kim HK, Kim YT, Sung SW, Park JD, Kang CH, Kim JH, Kim YJ. Management of congenital tracheal stenosis. Eur J Cardiothorac Surg. 2004. 25:1065–1071.
15. Backer CL, Mavroudis C, Dunham ME, Holinger L. Intermediate-term results of the free tracheal autograft for long segment congenital tracheal stenosis. J Pediatr Surg. 2000. 35:813–818. discussion 8-9.

16. Backer CL, Mavroudis C, Gerber ME, Holinger LD. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg. 2001. 19:777–784.

17. Grillo HC. Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg. 1994. 58:613–619. discussion 9-21.

18. Grillo HC, Wright CD, Vlahakes GJ, MacGillivray TE. Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg. 2002. 123:145–152.

19. Macchiarini P, Dulmet E, de Montpreville V, Mazmanian GM, Chapelier A, Dartevelle P. Tracheal growth after slide tracheoplasty. J Thorac Cardiovasc Surg. 1997. 113:558–566.

20. Anton-Pacheco JL, Cano I, Comas J, Galletti L, Polo L, Garcia A, Lopez M, Cabezali D. Management of congenital tracheal stenosis in infancy. Eur J Cardiothorac Surg. 2006. 29:991–996.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download