Abstract
This study was designed to investigate the effects of polyamines on mechanical contraction and voltage-dependent calcium current (VDCC) of guinea-pig gastric smooth muscle. Mechanical contraction and calcium channel current (IBa) were recorded by isometric tension recording and whole-cell patch clamp technique. Spermine, spermidine and putrescine inhibited spontaneous contraction of the gastric smooth muscle in a concentration-dependent manner. Spermine (2 mM) reduced high K+ (50 mM)-induced contraction to 16±6.4% of the control (n=9), and significantly inhibited IBa in a reversible manner (p<0.05; IC50=0.8 mM). Pre- and post-treatment of tissue with spermine (2-5 mM, n=10) also inhibited acetylcholine (10 µM)-induced phasic contraction to 5±6.4% of the control. Inhibitory effect of spermine on IBa was observed at a wide range of test potentials of current/voltage (I/V) relationship (p<0.05), and steady-state activation of IBa was shifted to the right by spermine (p<0.05). Spermidine and putrescine (1 mM each) also inhibited IBa to 51±5.7% and 81±5.3% of the control, respectively. And putrescine (1 mM) inhibited IBa at whole tested potentials (p<0.05) without significant change of kinetics (p<0.05). Finally, 5 mM putrescine also inhibited high K+-induced contraction to 53±7.1% of the control (n=4). These findings suggest that polyamines inhibit contractions of guinea-pig gastric smooth muscle via inhibition of VDCC.
The natural polyamines are aliphatic molecules widely distributed in prokaryotic and eukaryotic cells (1) and spermine, spermidine and putrescine their precursor, are natural constituents of eukaryotic cells. Putrescine is formed by decarboxylation of amino acid ornithine, and then converted to spermidine and spermine after condensation with aminopropyl groups derived from S-adenosyl-L-methionine. In addition to endogenous synthesis, polyamines can easily be obtained from many kinds of food (2). They are readily taken up by the gut and then enter the systemic circulation (3). Actually, all cells possess a transport system for the uptake of polyamines and its activation is known to depend on cell surface proteoglycans (4). There are reports that polyamines may act on both sides of membrane. Therefore, polyamines are expected to play certain roles in cellular processes.
It was suggested that polyamines are associated with growth, cell differentiation and proliferation in prokaryotic and eukaryotic cells (5). However, it has also been reported that polyamine content vary during cell cycle, increase in cancer (6), and decrease with aging (2). The intracellular concentrations of polyamines are in the millimolar ranges. But there are considerable differences among cells and tissues. These differences of intracellular concentrations also seems to imply multiple role of polyamines in cellular events, both physiological and pathological. Polyamines were found in different types of muscles and were shown to affect membrane excitability and motility (7-9). Generally, polyamines inhibited smooth muscle contractions (10-13): For example, spermine caused relaxation of vascular smooth muscles of guinea-pig in vitro and decreased vascular resistance in dog (14), and it also inhibited spontaneous contractions of rat uterus (11) and small intestine (15). Administration of polyamines into gastrointestinal (GI) tract was found to inhibit GI motility, resulting in the inhibition of gastric emptying in vivo (16). Besides, ingestion of polyamines inhibited gastric ulcer (17) and stimulated maturation of intestine in rats (18).
Hashimoto et al. (11) showed that increase of the extracellular concentration of Ca2+ counteracts the relaxation induced by spermine in uterus, suggesting that spermine interferes with the entry of Ca2+ through the cell membrane. In addition, the effects of spermine on intracellular Ca2+ and contractile force in intact intestinal smooth muscle were found to be strongly dependent on the degree of depolarization of the cell membrane. In their experiments, spontaneous contractions and contractions elicited by a moderately elevated K+ were potently inhibited by spermine but those by high concentration (120 mM) of K+ were hardly affected (13, 19). These results raise a possibility that the most plausible underlying mechanism by which spermine and spermidine relax smooth muscle is the inhibition of voltage-dependent Ca2+ channel (VDCC). In fact, polyamines have been proposed as natural calcium antagonists (14), and modulators of calcium channel conductance (20).
Dihydropyridine-sensitive voltage-dependent 'L-type' Ca2+ channels (VDCCL) have been described in most excitable tissues, including guinea-pig gastric circular myocyte (18, 21), and VDCCL are known to play a central role in the regulation of intracellular Ca2+ ([Ca2+]i) in smooth muscles (22). VDCCL of GI smooth muscles are essential prerequisite for the generation of spontaneous contraction and agonist induced contraction such as acetylcholine (ACh) (23, 24). Generally, VDCCL in smooth muscles are regulated by numerous humoral factors like various neurotransmitters as well as mechanical stretch (cytoskeleton) and related cytosolic factors (25, 26). Particularly, neuropeptide and neurotransmitters such as ACh and norepinephrine (NE) are well known to regulate Ca2+ current of smooth muscle, including GI smooth muscle (25, 27, 28). Effects of polyamines on the regulation of VDCCL have been reported inhibitory in small intestine. However, physiological activities of GI tract are very different, depending on the site (29, 30). For example, major function of stomach is storage and grinding of food, and that of small intestine absorption of nutrients. Accordingly patterns of motilities of two organs are absolutely different, implying that complicated mechanisms are involved in the regulation of each part of GI motility. Therefore, the identification of regulatory factors of VDCC in each part of GI tract and elucidation of its mechanism appear to be important. This study was undertaken to elucidate the role of polyamines in gastric antral contraction, especially the relationship between regulation of VDCCL and gastric antral contraction by polyamines in guinea-pig gastric antral smooth muscle.
Guinea-pigs of either sex, weighing 300-350 g, were exsanguinated after stunning. The antral portion of stomach was cut, and the mucosal layer was separated from the muscle layers in Ca2+-free physiological salt solution (Ca2+-free PSS). The circular muscle layer was dissected from the longitudinal layer using fine scissors and made into small segments (2×3 mm). These segments were incubated in Ca2+-free PSS for 30 min at 4℃, and they were then incubated for 15-25 min at 35℃ in the digestion medium containing 0.1% collagenase (Wako Pure Chemicals, Osaka, Japan), 0.05% dithiothreitol, 0.1% trypsin inhibitor and 0.2% bovine serum albumin. After the digestion, the supernatant was discarded, and the softened muscle segments were transferred into modified Kraft-Brühe (K-B) medium (31) and single cells were then dispersed by gentle agitation with a wide-bore glass pipette. Isolated gastric myocytes were kept in K-B medium at 4℃ until use. All experiments were carried out within 8 hr of harvesting cells and performed at room temperature.
Isolated cells were transferred to a small chamber on the stage of an inverted microscope (IMT-2, Olympus, Japan). The chamber was perfused with PSS (2-3 mL/min). Glass pipettes with a resistance of 2-5 MΩ were used to make a giga seal of 5-10 GΩ. Standard patch clamp techniques were used (32). An axopatch-1C patch-clamp amplifier (Axon instruments, CA, U.S.A.) was used to record membrane currents, and command pulses were applied by using IBM-compatible AT computer and pClamp software v.5.5.1. The data were displayed on a digital oscilloscope and a computer monitor.
Vertical (25 mL) chambers were used for the mechanical experiment. For the measurement of mechanical contractions, muscle strips (5×10 mm) from the antral tissue with circular direction were prepared in isometric contractile measuring system. In this system, one end of tissue was tied tightly to fixed holder and the other side was also linked by hook type of holder to force transducer (Harvard, Massachusetts, U.S.A.). The external solutions were changed with solutions which had previously been incubated (bubbled with 5% CO2/95% O2, 36℃) in water bath before application. All experiments were performed in the presence of a muscarinic receptor antagonist (1 µM atropine, ATR), adrenoceptor antagonists (1 µM phentolamine and 1 µM propranolol) as well as nerve blocker (1 µM tetrodotoxin, TTX) to elucidate the direct effect of polyamines on guinea-pig gastric smooth muscle.
Ca2+-PSS, containing (in mM): NaCl 135, KCl 5, CaCl2 1.8, MgCl2 1, glucose 10, and HEPES (N-[2-hydroxyethyl] piperazine-N'-[2-ethanesulphonic acid]) 10, was adjusted to pH 7.4 with NaOH. Modified K-B solution, containing (mM) L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2 3, glucose 10, HEPES 10, ethylene glycol-bis (2-aminoethyl ether- N,N,N',N'-tetraacetic acid (EGTA) 0.5, was adjusted to pH 7.4 with KOH. Pipette solution, containing (mM) CsCl 110, TEA 20, EGTA 10, HEPES10, Na2ATP 3, MgCl2 3.5, was adjusted to pH 7.3 with TRIZMA or CsOH. CO2/bicarbonate-buffered Tyrode solution contained (in mM) NaCl 122, KCl 4.7, MgCl2 1, CaCl2 2, NaHCO3 15, KH2PO4 0.93, and glucose 11 (pH 7.3-7.4, bubbled with 5% CO2/95% O2). Equimolar concentration of Na+ was replaced by K+ for making high K+ (50 mM) solution. All drugs used in this study were purchased from Sigma (St. Louis, MO, U.S.A.).
Changes of relative contraction by polyamines were analyzed by measuring the amplitude of spontaneous contraction in the presence and absence of polyamines. In the case of high K+ contraction, the amplitude of sustained contraction produced by high K+ (50 mM) in the presence of polyamines was compared to that of high K+ (50 mM) alone. The data are expressed as means±SEM. Statistical significance was estimated by paired and unpaired Student's t-test. p<0.05;0.05 was considered to be statistically significant.
Fig. 1 shows structures of the endogenous polyamines putrescine, spermidine and spermine. Effect of spermine and putrescine on isometric contraction of guinea-pig gastric antral smooth muscle was studied in the presence of nerve blockers (see Material and Methods). As shown in Fig. 2A, spermine inhibited spontaneous contraction of the muscle in a concentration-dependent and reversible manner. However, the frequency of contraction was not significantly changed (p>0.05). The spontaneous contractions were reduced to 81±3.0, 69±4.1, 49±4.9, 36±4.6, 26±3.1, and 7±2.4% of the control at 0.1, 0.2, 0.5, 1, 2, and 5 mM of spermine, respectively (n=8, 10, 10, 11, 15, and 8, respectively). However, 10-7 and 2×10-5 M spermine did not show inhibitory effect. IC50 of inhibition of contraction was 0.6±0.05 mM (Fig. 2B). In Fig. 2C, effects of putrescine on the spontaneous contraction of guinea-pig gastric antral smooth muscle are summarized. Putrescine also inhibited the spontaneous contraction, and the inhibitory effect was reversible. The spontaneous contractions were reduced to 97±1.3, 82±5.5, 64±9.5, 46±0.9, 3±1.0, and 2±1.0% of the control at 1, 2, 5, 10, 20, and 30 mM putrescine, respectively (n=5, 4, 4, 6, 7, and 5, respectively), and IC50 for inhibition of contraction was 9.7 mM (Fig. 2C).
To verify whether spermine and putrescine reduced the spontaneous contraction through inhibition of Ca2+ influx, the effects of spermine and putrescine on high K+ (50 mM)-induced contraction were studied. High K+ solution (50 mM) in the presence of nerve blockers produced biphasic contraction (an initial transient contraction followed by a sustained tonic contraction), which was reduced to 16±6.4% (n=9) by pretreatment of the muscle with 2 mM spermine (Fig. 3A, B). As shown in Fig. 3C, high K+-induced contraction was also reduced to 53±7.1% by pretreatment of the muscle with 5 mM putrescine (n=4).
As shown in Fig. 4A, ACh (10 µM) produced transient initial and tonic contraction which followed by a sustained phasic contraction. ACh (10 µM)-induced initial, tonic, and phasic contractions were 1,462±317.2 mN, 561±179.9 mN and 1,138±313.8 mN, respectively (n=20, Fig. 4B). In Fig. 4C, effect of nicardipine (2 µM) on ACh-induced phasic contraction was studied. As shown in Fig. 4C, D, ACh (10 µM) produced phasic contraction and it was completely inhibited to 2±1.0% of the control by post application of nicardipine 2 µM (n=6). Spermine (2 mM) also inhibited ACh (10 µM)-induced phasic contraction to 5±1.4% of the control (n=10; Fig. 4E, F). In Fig. 5, repetitive application of ACh (10 µM) produced contraction in guinea-pig gastric smooth muscle. Pretreatment of the muscle with 5 mM spermine reduced the ACh (10 µM)-induced initial and phasic contractions to 47±8.1 and 1±0.5% of the control, respectively (n=6, repectively; Fig. 5). These findings indicate that polyamine-induced relaxation is associated with the inhibition of Ca2+ influx through VDCC.
Membrane potential was held at -80 mV, and step depolarizing pulse to 0 mV for 420 msec was applied to cells at every 15 sec to record IBa. Extracellular Ca2+ was replaced by 10 mM Ba2+ after whole cell configuration was done. Effects of spermine on IBa at 0 mV were studied before and after application of spermine after the peak level of IBa reached steady level. Spermine (1 mM) decreased IBa at 0 mV in a reversible manner, and representative raw current traces are shown in Fig. 6A. In Fig. 6B, peak values of IBa were plotted as a function of time during the application of spermine: Reversible and inhibitory effect of spermine on IBa is shown. In Fig. 6C, effects of 1 mM each of spermine, spermidine and putrescine on IBa at 0 mV were studied and compared to each other before and after the application: Peak amplitude of IBa at 0 mV was decreased to 46±1.8, 51±5.7, and 81±5.3%, respectively, of the control in a reversible manner. Concentration-response relationships of spermine and putrescine on peak value of IBa at 0 mV were studied, and the result is summarized in Fig 6D, E: The peak values at 0 mV were significantly decreased to 72±10.8, 58±8.8, 46±1.7, 29±5.7, 7±2.6, and 0% by 0.1, 0.5, 1, 2, 5, and 10 mM spermine, compared to the control, respectively (mean±SEM, n=9, 6, 12, 7, 4, and 5, respectively; p<0.05; IC50=0.8 mM; Fig. 6D). And peak currents of IBa were significantly decreased to 81±5.3, 56±13, 41±5.4, 6±0.6, and 2±2% of the control at 1, 5, 10, 20, and 30 mM putrescine, respectively (n=9, 5, 5, 5, and 5, respectively; p<0.05; IC50=8 mM; Fig. 6E).
I/V relationship of IBa was also studied in the absence and presence of spermine and putrescine. The membrane potential was held at -80 mV, and 10 mV step depolarization, ranging from -40 mV to +50 mV, was applied to the cell for 420 ms before and after the application of spermine and putrescine. One mM spermine and putrescine were found to decrease IBa at membrane potential range of -20~+50 mV tested (n=5 and 6, respectively; p<0.05).
A modified double-pulse protocol was used to measure the steady-state inactivation of IBa as a function of membrane potentials. The prepulse potential, ranging from -100 to +20 mV, was applied for a duration of 3.75 sec. Following 7 msec interpulse interval at a potential of -80 mV, the membrane potential was raised to a test potential of 0 mV for 1 sec. The currents were then normalized to the current obtained at -100 mV (I/Imax) and plotted against each prepulse potential. Plotted data were well fitted by a Boltzmann equation, with a half-inactivation voltage (V0.5) of -31±1.3 mV in the control and -33±1.5 mV in the spermine treated groups (n=6), and slope factor (κ) of -11±0.8 in the control and -12±1.5 in the spermine treated cells (n=6; Fig. 7C; p>0.05). Steady-state activation curves were obtained from the I/V relations of IBa in the presence and absence of 1 mM spermine, and the peak conductance at each potential was calculated by using the following equation: IBa=gBa×(V-Erev) where gBa, V, and Erev are peak conductance, test potential and observed reversal potential, respectively. The values of half-activation were -9±0.5 mV and -6±0.6 mV (n=6; Fig. 4c; p<0.05) with slope factors (κ) of 5±0.2 and 5±0.1 in the control and spermine treated groups, respectively (n=6; Fig. 7C; p>0.05). In the case of putrescine (1 mM), it did not show any significant effect on kinetics of IBa, such as steady state activation and inactivation kinetics, in guinea-pig gastric myocytes (n=5, p>0.05; Fig. 7D).
In this study: 1) spermine and putrescine produced relaxation and inhibited high K+ (50 mM)- and ACh (10 µM)-induced contractions, and 2) spermine-induced inhibition of contraction was mediated by inhibition of VDCCL as can be seen in its rightward shifting of half-activation values in guinea-pig gastric myocytes.
Relaxation effect of polyamines has been reported in several smooth muscles, including uterus, trachea and ileum (10, 11, 20). In guinea-pig ileum, spermine and spermidine decreased the [Ca2+]i and caused relaxation by a mechanism that has been shown to involve the inhibition of the current through VDCCL in whole-cell voltage clamp experiment (20). In this study, we also found the inhibitory effect of polyamines on spontaneous contractions in guinea-pig gastric smooth muscles (Fig. 2). As seen in Fig. 2, spermine and putrescine produced relaxation of the smooth muscle in a concentration-dependent and reversible manner. And spermidine also reduced spontaneous contractions in a concentration dependent manner (n=4, IC50=1.1 mM; data not shown). Furthermore, high K+ (50 mM)-induced contractions were inhibited to 16% (n=9) and 53% (n=4) of the control by pretreatment of the muscle with spermine (2 mM) and putrescine (5 mM), respectively (Fig. 3). Post-application of spermine and putrescine also showed similar inhibitory effect on high K+ (50 mM)-induced contractions (n=4, data not shown), and 1 mM spermidine also suppressed high K+ (50 mM)-induced contractions to 28±4% of the control (data not shown, n=4). Since high K+ condition is known to produce contraction via membrane depolarization which is associated with activation of VDCCL, the inhibition of VDCCL seemed most probable underlying mechanism of relaxation by polyamines in guinea-pig gastric smooth muscle. In addition, ACh (10 µM)-induced contractions was also inhibited by spermine. Spermine decreased ACh (10 µM)-induced initial transient and later phasic contraction to 47% and 1% of the control, respectively. ACh-induced initial transient and post-phasic response were reported responsible for InsP3-induced Ca2+ release (IICR) and Ca2+ influx through VDCCL in GI smooth muscle, respectively (24). Taken together it is suggested that polyamines have an inhibitory effect by inhibiting VDCCL. In the experiment about inhibitory effect of spermine on ACh-induced initial contraction, the inhibition of IICR seemed to be underlying mechanism (8). Meanwhile, 1 and 2 mM putrescine were found to decrease spontaneous contraction to 82% and 81% of the control, respectively (Fig. 2C, 6C, E, 7B). The potency for the inhibition of high K+-induced contractions in guinea-pig gastric smooth muscle was much higher than that of rat uterus (IC50=13.7).
With the above backgrounds, we investigated the effect of polyamines on VDCC of guinea-pig gastric myocytes. As shown in Fig. 6A, B, spermine significantly inhibited IBa in a concentration-dependent and reversible manner, and this inhibitory effect of spermine was maintained over the whole range of I/V relationship (Fig. 7A). These findings are the same results as those observed in intestinal smooth muscle of guinea-pig: Gomez and Hellstrand (14) reported that IBa in guinea-pig intestinal smooth muscle was inhibited by spermine in a reversible manner (IC50=0.8 mM). In guinea-pig taenia coli, the relaxing effect of polyamines was associated with decreased intracellular free Ca2+ resulting from the inhibition of Ca2+ influx (12). Therefore, polyamines seem to inhibit Ca2+ conductance through VDCC, hence inhibiting contractions of GI tract.
The effect of polyamines on steady-state activation and inactivation curve of IBa is shown in Fig. 7C, D. As shown in Fig. 7C, spermine significantly shifted half-activation values to the right (Fig. 3B, p<0.05). In contrast, putrescine inhibited IBa in guinea-pig gastric myocytes without affecting steady-state activation and inactivation curves (Fig. 7D). Since polyamines are positively charged molecules, screening by polyamines of the cell membrane charge, which affects the gating properties of ionic channels, should be considered (42). However, several points are against this possibility. Reversal potentials of IBa in I/V relationship were not changed by polyamines. And inhibitory effects of polyamines were rapid and completely reversible. Therefore, nonspecific charge-screening effects are unlikely to be responsible for the inhibition of IBa. In the case of guinea-pig intestinal smooth muscle, VDCCL was inhibited without affecting kinetics (14, 20). Therefore, inhibitory mechanisms of VDCCL by polyamines in gastric smooth muscle seem to be different from that of intestinal smooth muscle.
As shown in Fig. 2C, 3C, putrescine inhibited spontaneous contraction and high K+ (50 mM)-induced contractions in guinea-pig gastric smooth muscle. And 1 mM putrescine also decreased IBa by 19% of the control in a reversible manner (Fig. 6C, E, 7B). However, Gomez and Hellstrand (14) could not observe any inhibitory effect of 1 mM putrescine on IBa in guinea-pig intestinal smooth muscle. Generally, spermine and spermidine have been known to inhibit contractile activities in smooth muscle (11, 12, 15, 34). However, the contractile effects of putrescine remain rather controversial; some report a slight inhibition and/or potentiation, and others report no significant effects (12, 15, 19). To our best knowledge, this study is the first report showing that putrescine inhibits contraction and IBa in GI smooth muscle. From these findings it is likely that polyamines have different roles even within GI tract, and their regulation of GI motilities is dependent on the type of tissues. Unfortunately, however, we do not know the reasons of why larger polyamine such as spermine inhibits VDCCL and contraction more effectively in guinea-pig gastric smooth muscle, whereas smaller polyamine like putrescine is ineffective in regulating contraction and IBa in small intestine and colon. Schoemaker (35) suggested different binding affinity of polyamines on VDCCL in rat brain: Binding affinity of polyamines on VDCCL might be different, depending on the types of polyamines and tissues.
Since polyamines are synthesized inside the cell, the sites of action of these substances under physiological conditions are regarded to be mainly intracellular side (36). Taenia coli contains about 160 nM of spermine per g of tissue, which corresponds to an intracellular concentration of about 0.2 mM/L. Estimated intracellular concentrations of polyamines in guinea-pig intestinal cells are reported in these ranges (8). However, distribution of spermine inside the smooth muscle cell is most likely not even, but rather concentrated in subcellular structures such as membrane, where the concentration would be much higher (13). In this study, we applied various concentrations of polyamines to the outside of the membrane in guinea-pig gastric smooth muscle, and observed relaxation of muscle and inhibition of IBa, which was similar to the results by others. As seen in Fig. 2C, 6E, the effect of extracellular application of polyamines on the contraction and IBa was rapid and reversible, suggesting that the compound does not necessarily have to enter inside the cell to block channel activity, although an intracellular site of the action might exist as well. In fact, there are also reports that polyamines may act on both inside and outside of the membrane (19, 20).
In summary, this study provides evidences that polyamines decreased IBa in concentration-dependent manner and also induced relaxation in guinea-pig gastric smooth muscle.
Figures and Tables
Fig. 1
Structures of the endogenous polyamines. The structures of polyamines putrescine, spermidine and spermine are shown in A-C.
Fig. 2
Effect of polyamines on spontaneous contraction in guinea-pig gastric smooth muscle. Effects of spermine and putrescine on isometric contraction were studied by using vertical chamber system. Spermine produces relaxation in a concentration-dependent manner (A). In (B) and (C), concentration-response relationship of spermine and putrescine are summarized. Relative contractions at various concentrations of spermine and putrescine are plotted and fitted by the non-linear regression equation.

Fig. 3
Relationship between polyamines-induced relaxation and Ca2+ influx through VDCC. High K+ solution (50 mM) in the presence of nerve blockers produced biphasic contraction in guinea-pig gastric smooth muscle. Such high K+ (50 mM)-induced contraction is reduced by pretreatment of the cells with 2 mM spermine in (A). Pannel (B) shows summarized data of all tissues tested. In (C), Inhibitory effect of putrescine (5 mM) on high K+ (50 mM)-induced contraction is also summarized.

Fig. 4
Inhibitory effect of spermine on acetylchole (ACh)-induced contraction through modulation of VDCC. (A) 10 µM ACh produces transient initial and tonic contraction which is superimposed by phasic contraction in a reversible manner. ACh-induced contraction are summarized in (B). ACh-induced phasic contraction is inhibited by nicardipine 2 µM in (C). Pannel (D) shows summarized data of all tissues tested. Effect of spermine on ACh-induced phasic contraction was also studied. Spermine (2 mM) inhibited ACh-induced phasic contraction in (E). And (F) shows summarized data of all tissues tested.
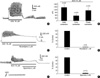
Fig. 5
Inhibition of ACh-induced contraction by spermine in guinea-pig gastric smooth muscle. (A) Repetitive application of ACh (10 µM) in guinea-pig gastric smooth muscle produces contraction. Pretreatment of the muscle with 5 mM spermine reduced ACh-induced initial contraction and inhibited phasic contraction in a reversible manner. Pannel (B) shows summarized data of all tissues tested.

Fig. 6
Effect of polyamines on IBa in guinea-pig gastric myocytes. IBa was recorded under the condition in which extracellular Ca2+ was replaced by 10 mM Ba2+. IBa is inhibited by the application of spermine in (A) and (B). In (C), effects of polyamines (1 mM) on IBa at 0 mV were studied. IBa is decreased by the application of 1 mM each of spemine, spermidine and putrescine. (D, E) Concentration-response relationship of spermine and putrescine on IBa is summarized. Normalized responses by various concentrations of spermine and putrescine against the peak values obtained at 0 mV are plotted and fitted by the non-linear regression equation.

Fig. 7
Effects of polyamines on I/V relationship and kinetics of IBa in guinea-pig gastric myocytes. In (A) and (B), I/V relationship of IBa by spermine and putrescine in guinea-pig gastric myocytes are shown. Averaged responses of IBa in the presence and absence of spermine and putrescine are plotted. (C) and (D) show steady-state activation and inactivation curves for the cells exposed to none and polyamines. In (C), steady-state activation and inactivation curves for the cells exposed to none (▴,●) and spermine (▵,○) are shown. Spermine shifted values of half-activation to rightward (n=5, p<0.05). (D) shows steady-state activation and inactivation curves for the cells exposed to none (▴,●) and putrescine (▵,○). There is no significant difference in values of half-activation and half-inactivation (n=5, p>0.05).

References
1. Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem Biophys Res Commun. 2000. 271:559–564.

2. Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006. 139:81–90.

3. Wery I, Kaouass M, Deloyer O, Buts JP, Barbason H, Dandrifosse G. Exogenous spermine induces maturation of the liver in suckling rats. Hepatology. 1996. 24:1206–1210.

4. Belting M, Persson S, Fransson LA. Proteoglycan involvement in polyamine uptake. Biochem J. 1999. 338:317–323.

6. Nilsson BO, Gomez M, Swärd K, Hellstrand P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle-implications for cellular growth and contractility. Acta Physiol Scand. 2002. 176:33–41.
7. Swärd K, Nilsson BO, Hellstrand P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea-pig ileum. Am J Physiol. 1994. 266:C1754–C1763.
8. Tsvilovskyy VV, Zholos AV, Bolton TB. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br J Pharmacol. 2004. 143:968–975.

9. Chideckel EW, Fedan JS, Mike P. Polyamines and putrescine relax respiratory tract smooth muscle in the guinea-pig. Eur J Pharmacol. 1985. 116:187–190.
10. Hashimoto H, Unemoto T, Hayashi M. Inhibitory action of spermine on the contractions of rat uterus. Am J Physiol. 1973. 225:743–746.

11. Maruta K, Mizoguchi Y, Osa T. Effects of polyamines on the mechanical and electrical activities of the isolated circular muscle of rat uterus. Jpn J Physiol. 1985. 36:903–915.

12. Nilsson BO, Hellstrand P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol Scand. 1993. 148:37–43.

13. Chideckel EW, Dedhia HH, Fedan JS, Teba L, Jain A. Spermine decreases peripheral vascular resistance in the dog and relaxes the isolated aorta of the guinea pig. Cardiovasc Res. 1986. 20:931–934.

14. Gomez M, Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995. 430:501–507.

15. Tansy MF, Martin JS, Landin WE, Kendall FM, Melamed S. Spermine and spermidine as inhibitors of gastrointestinal motor activity. Surg Gynecol Obstet. 1982. 154:74–80.
16. Aihara H, Otomo S, Isobe Y, Ohzeki M, Igarashi K, Hirose S. Polyamine inhibition of gastric ulceration and seretion in rats. Biochem Pharmacol. 1983. 32:1733–1736.
17. Martin JS, Tansy MF. Comparison of the inhibitory effects of spermine, papaverine and adrenalin upon isolated segments of the rat small intestine. Clin Exp Pharmacol Physiol. 1986. 13:87–90.
18. Peulen O, Deloyer P, Dandrifosse G. Short-term effects of spermine ingestion on the small intestine: a comparison of suckling and weaned rats. Reprod Nutr Dev. 2004. 44:353–364.

19. Fernandez AI, Cantabrana B, Sanchez M, Hidalgo A. Extracellular and intracellular effects of polyamines on smooth muscle contractions. Life Sci. 1995. 57:855–861.
20. Gomez M, Hellstrand P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch. 1999. 438:445–451.
21. Katzka DA, Morad M. Properties of calcium channels in guinea-pig gastric myocytes. J Physiol. 1989. 413:175–197.

22. Kim SJ, Ahn SC, Kim JK, Kim YC, So I, Kim KW. Changes in intracellular Ca2+ concentration induced by L-type Ca2+ channel current in guinea-pig gastric myocytes. Am J Physiol Cell Physiol . 1997. 8273:1947–1956.
23. Huang S, Nakayama S, Iino S, Tomita T. Voltage sensitivity of slow wave frequency in isolated circular muscle strips from guinea pig gastric antrum. Am J Physiol. 1999. 276:G518–G528.
24. Sato K, Sanders KM, Gerthoffer WT, Publicover NG. Sources of calcium utilized in cholinergic responses in canine colonic smooth muscle. Am J Physiol. 1994. 267:C1666–C1673.

25. Koh SD, Sanders KM. Modulation of Ca2+ current in canine colonic myocytes by cyclic nucleotide-dependent mechanisms. Am J Physiol. 1996. 271:C794–C803.
26. Xu WX, Kim SJ, Kim SJ, So I, Kang TM, Rhee JC, Kim KW. Effect of stretch on calcium channel currents recorded from the antral circular myocytes of guinea-pig stomach. Pflugers Arch. 1996. 432:159–164.

27. Kamimura N, Suga S, Wada J, Mio Y, Suzuki T, Wakui M. Excitatory and inhibitory actions of norepinephrine on the Ba2+ current through L-type Ca2+ channels of smooth muscle cells of guinea-pig vas deferens. J Cell Physiol. 1996. 169:373–379.
28. Wade GR, Barbera J, Sims SM. Cholinergic inhibition of Ca2+ current in guinea-pig gastric and tracheal smooth muscle cells. J Physiol. 1996. 491:307–319.
29. Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for intestinal pacemaker activity. Nature. 1995. 373:347–349.
30. Tomita T, Pang YW, Ogino K. The effects of nickel and cobalt ions on the spontaneous electrical activity, slow wave, in the circular muscle of the guinea-pig gastric antrum. J Smooth Muscle Res. 1998. 34:89–100.

31. Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by pre-incubation in a "K-B-medium". Pflugers Arch. 1982. 395:6–18.
32. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.
33. Hille B. Ionic channels of excitable membranes. 1992. 2nd. Mass: Sinauer, Sunderland;457–462.
34. Inoue Y, Xiong Z, Kitamura K, Kuriyama H. Modulation produced by nifedipine of the unitary Ba current of dispersed smooth muscle cells of the rabbit ileum. Pflugers Arch. 1989. 414:534–542.

35. Schoemaker H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium in rat brain. Eur J Pharmacol. 1992. 225:167–169.
36. Hougaard DM, Fujiwara K, Larsson LI. Immunocytochemical localization of polyamines in normal and neoplastic cells. Comparisons to the formaldehyde-fluorescamine and o-phthalaldehyde methods. Histochem J. 1987. 19:643–650.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download