Abstract
Bronchoplastic lobectomy is a lung-saving procedure indicated for central tumors, for which the alternative is pneumonectomy. We compared operative mortality and complications between bronchoplastic lobectomy and pneumonectomy in lung cancer patients. From March 1993 through December 2005, 1,461 patients were surgically resected for non-small cell lung cancer, including 73 who underwent bronchoplastic lobectomy and 258 who underwent pneumonectomy. Bronchoplastic lobectomy was performed on any lesion that could be completely resected by this technique, whereas pneumonectomy was only performed on lesions that could not be removed by bronchoplastic lobectomy. Operative deaths occurred in 1 of 73 (1.4%) bronchoplastic lobectomy and 26 of 258 (10.1%) pneumonectomy patients (p=0.014). Major complications occurred in 16 of 73 (21.9%) bronchoplastic lobectomy and 58 of 258 (22.5%) pneumonectomy patients (p=1.0). Bronchoplastic lobectomy has a lower risk of operative mortality than pneumonectomy. Although the complication rates were similar, bronchoplastic lobectomy was associated with improved postoperative cardiopulmonary status and a low prevalence of fatal complications after bronchial anastomosis. These findings indicate that bronchoplastic lobectomy is a valuable alternative to pneumonectomy for anatomically appropriate patients, regardless of underlying cardiopulmonary function.
Bronchoplastic procedures were initially designed for cancer patients unable to tolerate pneumonectomy, for those with low grade malignancies, or for the treatment of benign lesions. Despite substantial improvements in multimodality therapies, operative resection remains the most important element in curative therapy for lung cancer. Bronchoplastic lobectomy was first performed in 1947 (1) and was first applied to a lung cancer patient in 1952 to conserve lung parenchyma (2). This procedure altered the approach to selected malignant neoplasms and has gradually gained acceptance as the standard resection technique for lung cancer in elderly patients or patients with associated co-morbidity factors, in whom pneumonectomy would be hazardous (3, 4). However, bronchoplastic lobectomy was initially believed to carry a high risk due to bronchial anastomoses that can give rise to specific complications, including dehiscence, vascular fistula, and stenosis.
Although bronchoplastic lobectomy has been applied to lung cancer patients who could not tolerate pneumonectomy (3, 4), it has also been used in patients who could tolerate pneumonectomy (5, 6). Recent reports have suggested that bronchoplastic lobectomy should be used routinely in the management of patients with centrally located tumors, even in those with sufficient pulmonary reserve, and could achieve adequate cure rates (7, 8). To date, no randomized trials comparing bronchoplastic lobectomy with pneumonectomy have been performed, and they are unlikely to be performed. Thus, all available information is derived from unmatched, retrospective cohorts of patients. In the present study, to determine the feasibility and safety of bronchoplastic lobectomy in the treatment of respectable non-small cell lung cancer (NSCLC), we retrospectively compared rates of operative mortality and complications in patients treated with bronchoplastic lobectomy and pneumonectomy.
From March 1993 through December 2005, 1,461 consecutive patients clinically diagnosed with resectable NSCLC underwent surgical resections in a single institution, including 73 who underwent bronchoplastic lobectomy and 258 who underwent pneumonectomy. Of the 73 bronchoplastic lobectomy patients, full sleeve resection was performed on 56 and bronchoplasty with bronchial wedge excision was performed on 17. Patients underwent bronchoplastic lobectomy if their lesions could be completely resected by this technique, including patients with central tumors located at the origin of a lobar bronchus, patients with positive bronchial resection margin after standard lobectomy, and patients with N1 disease when both tumor and nodes could be completely resected. Patients underwent pneumonectomy only for lesions that could not be removed by bronchoplastic lobectomy. No attempt was made to match the groups, but they were fairly comparable in age, sex, histology, site, FEV1, stage, and nodal status.
All patients were staged according to the 1997 revisions in the international system for staging lung cancer. Operative mortality included 30 day mortality, all in-hospital mortality beyond 30 days, and mortality at home due to a cause strictly related to the operation. Complications were scored as anastomotic (bronchial) complications, specific surgical complications, and nonspecific surgical or medical complications.
All operations were carried out under general anesthesia with a double lumen endotracheal tube and through a posterolateral thoracotomy. A thoracic epidural infusion was used for postoperative analgesia. Bronchial anastomosis was performed using whole layer interrupted 4-0 absorbable polyfilament sutures, and a telescopic method was routinely used due to proximal and distal bronchial size discrepancy (luminal disparity). Inferior pulmonary ligament release maneuver was routinely performed, but pericardial release and bronchial anastomosis wrapping were not routine. Mediastinal lymphadenectomy was performed in all cases. Prior to closure of the thoracotomy, the anastomosis was checked with inflation pressures of 35-40 cm H2O. Intraoperative fiberoptic bronchoscopy was routinely used to assess the anastomosis at the end of operation.
Results are presented as mean±SD for continuous variables and as percentages for categorical variables. Qualitative data was compared using the chi-square test and differences between means were analyzed by Fisher's exact t-test. A p-value of 0.05 or less was considered statistically significant.
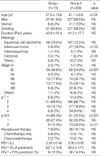
The bronchoplastic lobectomy group consisted of 67 men and 6 women, of mean age 57.5±13.6 yr (range, 13 to 77 yr). The pneumonectomy group consisted of 227 men and 31 women, of mean age 61.1±9.8 yr (range, 19 to 81 yr).
Histologic characteristics and postoperative pathologic staging for both groups are shown in Table 1. In the bronchoplastic lobectomy group, the rates of carcinoid and stage 1B tumors were high and the rate of adenocarcinoma was low compared with the pneumonectomy group.
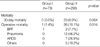
Bronchoplastic lobectomies were performed on 31 right upper lobes (42.5%), 18 left lower lobes (24.7%), 16 left upper lobes (21.9%), and 8 other lobes (10.9%), whereas pneumonectomies were performed on 114 right lungs (44.2%) and 144 left lungs (55.8%) (Table 2).
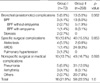
Postoperative mortality rates are shown in Table 3. There was a single operative mortality among the 73 patients who underwent bronchoplastic lobectomies (1.4%). This patient, a 68 yr old man with diabetes mellitus who underwent a coronary artery bypass graft prior to bronchoplastic right lower lobe lobectomy, died on postoperative day 58 of pneumonia and acute respiratory distress syndrome (ARDS) due to a postoperative bronchopleural fistula (BPF) of unknown cause that developed proximal to the anastomosis site.
In contrast, operative mortality occurred in 26 of the 258 patients (10.1%) who underwent pneumonectomy, a rate significantly higher than that observed in patients who underwent bronchoplastic lobectomy (p=0.014). The major cause of death in patients who underwent pneumonectomy was pulmonary complications, including pneumonia (46.2%) and ARDS (26.9%).
There were no significant difference in anastomotic (bronchial), specific surgical, and nonspecific surgical or medical complications between patients who underwent bronchoplastic lobectomy and those who underwent pneumonectomy (Table 4).
Surgical approaches to lung cancer vary from limited parenchymal resection for peripheral, small, and early-stage cancers to pneumonectomy for centrally located tumors with nodal involvement. Preoperative cardiopulmonary function and the amount of pulmonary parenchyma resected influence postoperative functional performance and likely affect postoperative quality of life (3, 7, 9). Therefore, individualization of technique is important for survival and quality of life outcomes.
Bronchoplastic lobectomy is a technically more demanding procedure than standard lobectomy and pneumonectomy, and any resulting bronchial anastomosis can give rise to specific complications. This is one reason that initial efforts at parenchyma-sparing operations focused on patients with limited pulmonary reserve who were not candidates for pneumonectomy (3, 4). Based on favorable experiences with such patients, the indications for bronchoplastic lobectomy expanded to include patients with anatomically favorable tumors regardless of their preoperative pulmonary status (5-8). We found that patients who underwent bronchoplastic lobectomy had lower rates of ARDS and pneumonia (1.4% and 6.8%, respectively) than those who underwent pneumonectomy (9.3% and 12.0%, respectively). Since preservation of lung parenchyma is believed to reduce the risks of operative mortality and should improve functional capacity and possible quality of life in the long term, it is logical that the incidence of respiratory failure was lower after bronchoplastic lobectomy.
Parenchymal sparing bronchoplastic lobectomy has several advantages compared with pneumonectomy. Postoperatively, mortality and morbidity should be lower (3, 10, 11), due to the avoidance of deleterious and procedure-specific complications of pneumonectomy, including pulmonary edema, bronchopleural fistula, and pneumonia, a finding supported by our results. The mortality rate following pneumonectomy has been reported to range from 4.1% to 10% (3, 10, 12, 16), whereas the mortality rate following bronchoplastic lobectomy ranged from 1.3% to 7% (3, 6, 8, 10-17). We observed mortality rates of 10.1% and 1.4%, respectively. Bronchoplastic lobectomy was initially believed to be a higher risk procedure, due to a theoretical increase in the risk of anastomotic complications, including dehiscence, vascular fistula, and stenosis (3, 4, 7, 9, 10, 14, 18). These complications, however, are rare, due to improved surgical techniques that preserve the bronchial blood supply, creation of a tension free bronchial anastomosis, and improved suture materials, all of which have resulted in better bronchial healing (4, 7, 12). Therefore, bronchoplastic lobectomy appears to be an alternative to pneumonectomy regarding operative mortality and morbidity (3, 7-10).
Regarding quality of life issues, lung-saving resections including sleeve or bronchoplastic lobectomy preserve greater respiratory capacity (19) than pneumonectomy and allow for radical resection of secondary lung malignancies (12, 14), which has increased in recent years. Mediastinal shifting and right ventricular dysfunction, the deleterious cardiovascular and hemodynamic consequences of pneumonectomy, resulted from increased pulmonary vascular resistance. These consequences led to decreased diffusion capacity and elevated pressures in the right side of the heart during exercise in most patients, progression of emphysema and development of pulmonary hypertension, resulting in cor pulmonale in some patients (11, 20).
Specific procedure-related complications of bronchoplasty were related with impaired anastomotic healing and retention of bronchial secretions. Anastomotic disruption and subsequent fistularization to the pulmonary artery have not been reported recently. The incidence of anastomotic dehiscence and BPF has been reported to range from 0% to 6% (3, 7, 9, 10, 12, 14), in agreement with our finding of 4.1%. Anastomotic strictures respond to a dual mechanism; that is, bronchial ischemia occurs following sleeve bronchoplasty, whereas, in cases of bronchial wedge excision, a too generous cartilaginous bridge may bulge into the lumen, causing an obstruction. The overall incidence of stenosis has been reported to range from 3% to 9% (7, 16), in agreement with our finding of 2.7%. The consequences of these complications on patient outcomes, however, are considerably less deleterious than the complications of pneumonectomy (3, 6, 8, 10, 11, 21).
For anatomic reasons, right upper lobectomy is most suitable for bronchoplasty. Of our 73 patients who underwent bronchoplastic lobectomy, 31 (42.5%) underwent right upper lobectomy, a rate similar to that reported by others (3, 12-15). The proximal transsection of the main stem bronchus is performed at the same level as for pneumonectomy. On the left side, the close anatomic location of the lobar take-offs narrows the resection margin, an additional drawback to conservative resection arises when the uppermost branches of the left pulmonary artery are involved by the tumor.
No prospective studies comparing bronchoplastic lobectomy and pneumonectomy have been conducted. Retrospective studies, however, have shown comparable mortality and survival rates for the two procedures. In our series, the operative mortality rate was 1.4% in the group of patients who underwent bronchoplastic lobectomies, compared with 10.1% for the group of patients who underwent pneumonectomies, whereas major complication rates were similar in the two groups (21.9% vs. 22.5%). Although the comparison of these two groups was retrospective and nonrandomized, these results suggest that bronchoplastic lobectomy is superior to pneumonectomy in selected patients. This may be due to the improved postoperative cardiopulmonary status after bronchoplastic lobectomy, owing to the preservation of lung parenchyma. Moreover, bronchial anastomosis can be performed with a low rate of complications.
In conclusion, our results indicate that bronchoplastic lobectomy is a valuable alternative to pneumonectomy for anatomically appropriate patients, regardless of their underlying cardiopulmonary function, owing to a substantially lower operative mortality rate.
References
1. Price-Thomas C. Conservative resection of the bronchial tree. J R Coll Surg Edinb. 1956. 1:169–186.
2. Allison PR. Course of thoracic surgery in Groningen. Ann R Coll Surg. 1954. 25:20–22.
3. Gaissert HA, Mathisen DJ, Moncure AC, Hilgenberg AD, Grillo HC, Wain JC. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg. 1996. 111:948–953.

4. Faber LP, Jensik RJ, Kittle CF. Results of sleeve lobectomy for bronchogenic carcinoma in 101 patients. Ann Thorac Surg. 1984. 37:279–285.

5. Faber LP. Sleeve lobectomy. Chest Surg Clin N Am. 1995. 5:233–251.
6. Suen HC, Meyers BF, Guthrie T, Pohl MS, Sundaresan S, Roper CL, Cooper JD, Patterson GA. Favorable results after sleeve lobectomy or bronchoplasty for bronchial malignancies. Ann Thorac Surg. 1999. 67:1557–1562.

7. Tedder M, Anstadt MP, Tedder SD, Lowe JE. Current morbidity, mortality, and survival after bronchoplastic procedures for malignancy. Ann Thorac Surg. 1992. 54:387–391.

8. Tronc F, Grégoire J, Rouleau J, Deslauriers J. Long-term results of sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg. 2000. 17:550–556.

9. Martin-Ucar AE, Chaudhuri N, Edwards JG, Waller DA. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg. 2002. 21:601–605.

10. Deslauriers J, Gregoire J, Jacques LF, Piraux M, Guojin L, Lacasse Y. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites of recurrences. Ann Thorac Surg. 2004. 77:1152–1156.
11. Okada M, Yamagishi H, Satake S, Matsuoka H, Miyamoto Y, Yoshimura M, Tsubota N. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg. 2000. 119:814–819.

12. Mehran RJ, Deslauriers J, Piraux M, Beaulieu M, Guimont C, Brisson J. Survival related to nodal status after sleeve resection for lung cancer. J Thorac Cardiovasc Surg. 1994. 107:576–583.

13. Van Schil PE, Brutel de la Rivière A, Knaepen PJ, van Swieten HA, Reher SW, Goossens DJ, Vanderschueren RG, van den Bosch JM. Long-term survival after bronchial sleeve resection: univariate and multivariate analyses. Ann Thorac Surg. 1996. 61:1087–1091.

14. Icard P, Regnard JF, Guibert L, Magdeleinat P, Jauffret B, Levasseur P. Survival and prognostic factors in patients undergoing parenchymal saving bronchoplastic operation for primary lung cancer: a series of 110 consecutive cases. Eur J Cardiothorac Surg. 1999. 15:426–432.

15. Fadel E, Yildizeli B, Chapelier AR, Dicenta I, Mussot S, Dartevelle PG. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg. 2002. 74:851–858.

16. Kim YT, Kang CH, Sung SW, Kim JH. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg. 2005. 79:1153–1161.

17. Cho SK, Sung KI, Lee C, Lee JI, Kim JH, Kim YT, Sung SW. Long term results of bronchial sleeve resection for primary lung cancer. Korean J Thorac Cardiovasc Surg. 2001. 34:917–923.
18. Terzi A, Lonardoni A, Falezza G, Furlan G, Scanagatta P, Pasini F, Calabro F. Sleeve lobectomy for non-small cell lung cancer and carcinoids: results in 160 cases. Eur J Cardiothorac Surg. 2002. 21:888–893.

19. Koh YM, Park SJ, Suh GY, Chung MP, Kim H, Kwon OJ, Rhee CH, Kim KM, Kim JG, Shim YM. Preservation of pulmonary function after sleeve lobectomy in patients with lung cancer. Tuberc Respir Dis. 1999. 47:35–41.

20. Burrows B, Harrison RW, Adams WE, Humphreys EM, Long ET, Reimann AF. The postpneumonectomy state: clinical and physiologic observations in thirty-six cases. Am J Med. 1960. 28:281–297.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download