Abstract
Behçet's disease (BD) is a systemic vasculitis involving diverse sizes of arteries and veins. We performed this study to evaluate the vascular changes by assessment of the arterial stiffness and intima-media thickness (IMT) of carotid artery in Korean patients with BD. Forty-one patients with BD and age-, and sex-matched 53 healthy subjects were recruited in this study. Carotid arterial stiffness and IMT were assessed by using high-resolution B-mode ultrasonography. Arterial stiffness parameters such as carotid arterial distensibility coefficient, stiffness index, and incremental elastic modulus (Einc) were significantly increased in BD patients compared with those in healthy subjects, but not in IMT. Positive relationship was noted between age and IMT, whereas age of onset was significantly associated with arterial stiffness in BD. This finding suggests impaired endothelial function before visible structural changes of arterial wall in BD. Age and age of onset may be an independent risk factor for carotid IMT and arterial stiffness, respectively. Further studies in more large populations are required to confirm our results.
Behçet's disease (BD) is a chronic, relapsing, multisystemic inflammatory disorder of unknown cause, which is mainly characterized by recurrent aphthous oral ulcers, genital ulcers, uveitis, and skin lesions (1). A wide spectrum of clinical features is observed, including involvement of the ophthalmic, musculoskeletal, vascular, central nervous, and gastrointestinal systems. Diverse vascular complications, such as deep vein thrombosis, myocardial infarction, arterial aneurysm, and arterial thrombus formation have been noted in patients with BD in about 20% to 35% of cases, predominantly in male patients and those with venous lesions (2). In general, BD patients with major vessel involvements have a poor prognosis.
The histopathological features are mainly characterized by vasculits, with prominent neutrophil and monocyte infilatration in perivascular lesions with or without fibrin deposition in the vessel wall (3). Although the pathogenic mechanism of vascular involvement in BD is under investigation, endothelial cell dysfunction is thought to play an important role in the development of these lesions (4-7). Endothelial dysfunction leading to abnormal coagulation or fibrolytic activity and impaired brachial artery flow-mediated dilatation has been demonstrated in BD. Because flow-mediated dilatation is endothelium-dependent and is largely controlled by the release of endothelial nitric oxide (NO), an impairment in endothelium-dependent flow-mediated dilatation suggests a decreased endothelial NO activity (8). This lack of activity may contribute to the vascular lesions often seen in BD. In addition, endothelial NO has been found to directly regulate large artery stiffness in vivo (9, 10).
The development of atherosclerotic changes in the vessel wall is initiated by perturbations in endothelial function, reflecting a functional change before the presence of morphologic changes. Although a variety of methods have been developed to evaluate endothelial function, non-invasive high-resolution B-mode ultrasonography is typically used. Arterial intima-media thickness (IMT) is a relatively sensitive marker of early atherosclerotic vessel wall changes, especially in the common carotid artery (11, 12). Arterial stiffness is another parameter which predicts cardiovascular risk that has been found to reflect the functional properties of arteries (12).
Acute systemic inflammation and chronic systemic vasculitis are noted as being associated with endothelial dysfunction (13). Moreover, inflammation is known to be an important risk factor for future cardiovascular events (14). These findings have led to the hypothesis that BD-associated acute and chronic inflammatory processes may cause endothelial dysfunction, leading to subsequent increases in IMT and arterial stiffness that are closely related to the clinical course of BD patients. Because geographic variations in clinical features including vascular lesions are significant, information has been limited regarding IMT and arterial stiffness in BD patients from different ethnic groups. In this study, we investigated carotid IMT and arterial stiffness in Korean patients with BD. We then assessed whether these predictive markers were affected by clinical parameters of BD.
This study included 41 patients with BD who fulfilled the International Study Group (ISG) criteria (15), along with 53 healthy controls matched to the patients for age, sex, blood pressure, heart rate, height, and total cholesterol and glucose levels. The frequency of smokers, if any, was also taken into account. Subjects with hypertension, diabetes mellitus, hyperlipidemia (total cholesterol <200 mg/dL), or a previous history of coronary artery disease or myocardial infarction were excluded. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or the current use of antihypertensive medications for diagnosed hypertension. The age of onset was defined as the time at which the patients had met the ISG criteria.
At examination, the presence of two or more of the following Behçet's clinical features was considered as active disease: oral ulceration, genital ulceration, skin lesions, ocular lesions, active major vessel disease, and active major organ involvement including active gastrointestinal or neurological lesions. During the course of the disease, the presence of one or more of the following clinical features defined severe disease (16): posterior uveitis or retinal vasculitis, gastrointestinal ulcerations with bleeding or perforation, major organ involvement, and major vessel involvement. In addition, BD patients with venous or arterial occlusive diseases or arterial aneurysm were considered as having vascular lesions; however, those with superficial thrombophlebitis were not considered as such. The duration of the disease in the BD group was calculated from the time from which the ISG criteria were fulfilled to the time of examination.
Using standard laboratory methods, the levels of total cholesterol and glucose were measured with fasting blood samples from all subjects. The study was approved by the Hospital Ethics Committee, and informed and written consent was obtained from each subject.
All subjects were asked to refrain from caffeine, alcohol, and smoking for 12 hr prior to the measurement. Ultrasound measurement of the common carotid artery was performed with an 8 MHz linear array transducer (Sequoia 512, Acuson, Mountain View, CA, U.S.A.) after resting in the supine position for 15 min. All measurements were performed by the same trained sonographer while in the supine position with the head slightly turned contralateral to the side being examined. The right and left CCA were scanned first in a transverse plane and were then scanned longitudinally. For the measurement of IMT of the common carotid artery, two images of the right CCA and two images of the left CCA from the bifurcation of the carotid artery were scanned at the peak of the R wave on the electrocardiogram (ECG) and were stored as digital images. For the measurement of the carotid systolic and diastolic diameters, B-mode images of three consecutive heart beats were recorded. After ultrasound examination, brachial blood pressure was measured from the left upper arm in a supine position using a semiautomatic device with oscillometric sensors.
Measurement of IMT was performed by computer with automatic IMT measurement software (M'Ath®-Std©, Metris, Argenteuil, France) (17). The IMT was measured 2 cm proximal to the carotid bifurcation along at least 1 cm of axial length, and was measured as the distance between the lumenintima interface and the media-adventitia interface using an automated edge detection algorithm. Measurements with a quality index ≥0.5 were used in the analysis. For analysis, the mean of the right and left CCA IMTs was used.
Systolic diameters were measured at the maximal diameter of the CCA, and diastolic diameters were measured at the peak of the R wave on the ECG. Measured diameters were averaged and were used for the calculation.
Functional arterial wall properties were assessed by distensibility coefficient (DC), stiffness index (β), and incremental elastic modulus (Einc) (18, 19). Carotid arterial DC, β, and Einc were calculated as follows:
Distensibility coefficient (DC, kPa-1×10-3)=2 (ΔD/DD)/(ΔP×0.133/1000)
β=ln (SBP/DBP)/(ΔD/DD)
Einc (kPa×103)=3 (1+LCSA/IMCSA)/DC
where ΔD=the difference between the systolic and diastolic diameters, DD=end-diastolic diameter, ΔP=pulse pressure (mmHg), SBP=systolic blood pressure, and DBP=diastolic blood pressure. LCSA (lumen cross sectional area) and IMCSA (intima-media cross sectional area) were calculated as LCSA=πDD2/4 and IMCSA=π(DD/2+IMT)2-π(DD/2)2.
Differences in non-continuous or continuous variables between groups were compared using the chi-square test or the independent t-test when indicated. Bivariate correlations between two continuous variables were evaluated using the Pearson correlation coefficient when indicated. Multiple regression analysis was performed to exclude confounding effects of clinical variables of BD and other risk factors on carotid IMT and arterial stiffness. p values less than 0.05 were considered statistically significant. The data were analyzed using the SPSS statistical package program version 11.5 for Windows (SPSS Inc., Chicago, IL, U.S.A.).
The clinical characteristics of the 41 patients with BD enrolled in this study are summarized in Table 1.
Table 2 shows the demographic features and laboratory findings for each group. No differences between patients with BD and controls were found in age, sex ratio, or other potential parameters that affect arterial stiffness and IMT, such as weight, height, smoking, heart rate, systolic and diastolic blood pressure, pulse pressure, plasma glucose, and total cholesterol levels.
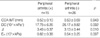
There were no significant differences in common carotid artery IMT values between patients with BD and control subjects, respectively (0.52±0.09 vs. 0.52±0.06, p=0.811) (Table 3). Carotid plague and aneurysm were not detected in BD patients or control subjects. We identified significant differences in all three arterial stiffness parameters between BD patients and control subjects, including DC (23.10±9.5 vs. 27.90±10.14, p=0.021),β(3.26±0.45 vs. 3.04±0.32, p=0.007), and Einc (0.64±0.33 vs. 0.49±0.16, p=0.008) (Table 3).
No associations between clinical, demographic, or laboratory parameters or carotid IMT were identified in BD patients. However, linear regression analysis showed that age and age of onset were positively associated with increased IMT (r=0.488, p=0.001 and r=0.328, p=0.036, respectively). The relationships between arterial stiffness indexes and clinical, demographic, and laboratory parameters from BD patients were analyzed using Pearson correlation analysis or t-test where indicated. BD with peripheral arthritis was significantly prone to increased arterial stiffness compared with those without peripheral arthritis (Table 4). Patients with peripheral arthritis exhibited a lower DC and higher β and Einc values than those without peripheral arthritis. In addition, a positive relationship between age of onset and arterial stiffness indices including DC, β and Einc was also noted in linear regression analysis (r=0.484, p=0.001, r=0.537, p<0.001, and r=0.487, p=0.002, respectively).
Multiple regression analysis for arterial stiffness indexes was used to adjust for potential confounding influences of age, sex, age of onset, and peripheral arthritis among the clinical features of BD. Age of onset of all arterial stiffness measures (DC, β, and Einc) had statistical significance (p=0.004, p=0,002, and p=0.006, respectively), as did in the study for carotid IMT (p=0.021) (Table 5).
Although the exact pathogenesis of BD remains unclear, small vessel vasculitis accounts for a considerable portion of the pathogenic processes in BD. In addition, large venous or arterial lesions can occur in up to one third of patients with BD (1). Despite uncertainty in the pathogenic mechanism of vascular lesions in BD, vascular endothelial dysfunction has been recognized in BD and is thought to play an important role in the vascular lesions (6, 7, 20, 21). Structural and functional features of the vascular system have also become an important issue in BD. In the present study, we measured the carotid IMT and rterial stiffness by means of noninvasive high resolution ultrasonography.
Previous ultrasonographic data for carotid IMT and/or arterial stiffness in Turkish study groups showed increased IMT and arterial stiffness in BD patients compared to controls (21, 22). Our study showed that the carotid IMT value in BD patients was not different from that in control subjects. However, arterial stiffness in BD patients was significantly higher than in control subjects. Several explanations for the lack of a difference in carotid IMT between the two groups can be considened. First, the general characteristics of patients enrolled in this study seem to be different from those of previous studies. In previous Turkish population studies, a difference in disease duration or age might influence the results between the studies (21, 22). Second, on the basis that arterial stiffness may be the first or an early manifestation of structural and functional vascular changes before the appearance of increased carotid IMT, there may be a certain potential of increased carotid IMT in BD patients compared with controls. Third, a multicenter epidemiologic study demonstrated that the pattern of clinical features in the Korean BD population were partially different from those in Middle East and Mediterranean regions. Severe manifestations, especially in vascular involvement and neurologic symptoms, appeared less frequently in Korean BD patients (23). We suppose that disease activity or severity in BD may affect the vascular wall changes, although a definite association has not been determined.
With respect to clinical features related with carotid arterial IMT, Alan et al. described that only traditional cardiovascular risk factors including SBP, DBP, and pulse pressure were identified as potential contributing factors for vascular changes such as carotid IMT in BD without observing unique risk factors associated with the disease alone (21). On the other hand, a Turkish study exhibited a weak positive association between common carotid IMT and disease duration (22). Our study identified that the carotid IMT level was related with patients' age, but not the duration of the disease.
A Turkish population study showed that SBP may be a risk factor related to arterial stiffness (21). In an arterial stiffness study using pulse wave velocity in Korean BD patients, Chang et al. demonstrated that age and mean arterial pressure may be independent risk factors for increased pulse wave velocity in BD (24). However, an association between increased arterial stiffness and age of disease onset was found in this study by ultrasonography. Although the presence of peripheral arthritis in BD showed an association with all arterial stiffness indices in univariate analysis, the statistical significance of peripheral arthritis was lost in multiple regression analysis.
In addition, no difference of systolic and diastolic diameters of CCA was identified between BD patients and controls in this study (data not shown). This finding may be due to several factors. First, the prevalence of vascular involvements, especially artery, in Korean BD seemed to be low, although the prevalence of these in BD was reviewed between 27.7 and 60.0% in western populations (25). Second, the prevalence of pulmonary or femoral arterial involvement was more predominant compared with carotid arterial involvement (26). Third, our data have limitations in their interpretation, since this study was performed in a small population.
A definite influence of characteristics of laboratory findings or serologic markers from BD on abnormalities in carotid IMT and arterial stiffness has not yet been determined, even though a great variety of potential factors including homocysteine, von Willebrand factor, and tissue plasminogen activator inhibitor may play important roles in the pathogenesis of endothelial dysfunction in BD (4, 5). Further investigation for a predictive laboratory marker related with vascular lesions is still required.
We did not perform evaluation of the involvement of other major arteries such as aorta, femoral artery, innominate artery, and brachial artery in this study. Carotid IMT has been shown to be associated with the extent of atherosclerosis in the aorta and coronary arteries (11, 12, 27, 28). However, carotid IMT of patients with BD in this study was not different from that of the control group. In addition, no carotid plaque was noted. These findings in this study suggest that the possibility of identifying advanced atherosclerotic lesions in other proximal or major arteries might not be high.
In conclusion, the present study demonstrated that patients with BD have significantly increased arterial stiffness compared to control subjects. However, this was not the case for carotid IMT, suggesting impaired endothelial function of carotid artery. Age was an independent significant factor associated with increased carotid IMT, whereas onset age was considered a risk factor for arterial stiffness in BD. Longitudinal studies in a large population are required to determine the prognostic implications of increased carotid arterial stiffness in BD.
Figures and Tables
Table 4
Arterial wall measurements of CCA according to the presence of peripheral arthritis in BD patients
References
1. Sakane T, Takeno M, Suzuki N, Inaba G. Behcet's disease. N Engl J Med. 1999. 341:1284–1291.
2. Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, Yurdakul S, Yazici H. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine (Baltimore). 2003. 82:60–76.
3. Jorizzo JL, Abernethy JL, White WL, Mangelsdorf HC, Zouboulis CC, Sarica R, Gaffney K, Mat C, Yazici H, al Ialaan A, Assad-Khalil SH, Kaneko F, Frederick Jorizzo EA. Mucocutaneous criteria for the diagnosis of Behcet's disease: an analysis of clinicopathologic data from multiple international centers. J Am Acad Dermatol. 1995. 32:968–976.
4. Hampton KK, Chamberlain MA, Menon DK, Davies JA. Coagulation and fibrinolytic activity in Behcet's disease. Thromb Haemost. 1991. 66:292–294.
5. Ozoran K, Dugun N, Gurler A, Tutkak H, Tokgoz G. Plasma von Willebrand factor, tissue plasminogen activator, plasminogen activator inhibitor, and antithrombin III levels in Behcet's disease. Scand J Rheumatol. 1995. 24:376–382.
6. Chambers JC, Haskard DO, Kooner JS. Vascular endothelial function and oxidative stress mechanisms in patients with Behcet's syndrome. J Am Coll Cardiol. 2001. 37:517–520.
7. Haznedaroglu IC, Ozcebe OI, Ozdemir O, Celik I, Dundar SV, Kirazli S. Impaired haemostatic kinetics and endothelial function in Behcet's disease. J Intern Med. 1996. 240:181–187.
8. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995. 91:1314–1319.

9. Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002. 105:213–217.

10. Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001. 38:1049–1053.

11. Simons PC, Algra A, Bots ML, Grobbee DE, van der Graaf Y. Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease). Circulation. 1999. 100:951–957.
12. Bots ML, Hofman A, De Jong PT, Grobbee DE. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann Epidemiol. 1996. 6:147–153.

13. Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, Donald AE, Palacios M, Griffin GE, Deanfield JE, MacAllister RJ, Vallance P. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000. 102:994–999.

14. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997. 336:973–979.

15. International Study Group for Behcet's Disease (ISGBD). ISGBD): Criteria for diagnosis of Behcet's disease. Lancet. 1990. 335:1078–1080.
16. Kim JU, Chang HK, Lee SS, Kim JW, Kim KT, Lee SW, Chung WT. Endothelial nitric oxide synthase gene polymorphisms in Behcet's disease and rheumatic diseases with vasculitis. Ann Rheum Dis. 2003. 62:1083–1087.

17. Touboul PJ, Vicaut E, Labreuche J, Belliard JP, Cohen S, Kownator S, Pithois-Merli I. Paroi Arterielle et Risque Cardiovasculaire Study Investigators. Design, baseline characteristics and carotid intima-media thickness reproducibility in the PARC study. Cerebrovasc Dis. 2005. 19:57–63.

18. Jourdan C, Wuhl E, Litwin M, Fahr K, Trelewicz J, Jobs K, Schenk JP, Grenda R, Mehls O, Troger J, Schaefer F. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005. 23:1707–1715.

19. Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Kamp O, Westerhof N, Bouter LM, Stehouwer CD. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003. 107:2089–2095.
20. Kayikcioglu M, Aksu K, Hasdemir C, Keser G, Turgan N, Kultursay H, Doganavsargil E. Endothelial functions in Behcet's disease. Rheumatol Int. 2006. 26:304–308.
21. Alan S, Ulgen MS, Akdeniz S, Alan B, Toprak N. Intima-media thickness and arterial distensibility in Behcet's disease. Angiology. 2004. 55:413–419.
22. Keser G, Aksu K, Tamsel S, Ozmen M, Kitapcioglu G, Kabaroglu C, Killi R, Bayindir O, Doganavsargil E. Increased thickness of the carotid artery intima-media assessed by ultrasonography in Behcet's disease. Clin Exp Rheumatol. 2005. 23:Suppl 38. 71–76.
23. Bang D, Lee JH, Lee ES, Lee S, Choi JS, Kim YK, Cho BK, Koh JK, Won YH, Kim NI, Park SD, Ahn HJ, Lee YW, Wang HY, Lee WW, Eun HC, Song ES, Lee SW, Lee CW, Lee CJ, Park JH, Song YW, Kim ST, Kim CY, Park JK, Kwon KS. Epidemiologic and clinical survey of Behcet's disease in Korea: the first multicenter study. J Korean Med Sci. 2001. 16:615–618.
24. Chang HK, Kim SK, Lee SS, Rhee MY. Arterial stiffness in Behcet's disease: increased regional pulse wave velocity values. Ann Rheum Dis. 2006. 65:415–416.

25. Koc Y, Gullu I, Akpek G, Akpolat T, Kansu E, Kiraz S, Batman F, Kansu T, Balkanci F, Akkaya S, Telatar H, Zileli T. Vascular involvement in Behcet's disease. J Rheumatol. 1992. 19:402–410.
26. Sarica-Kucukoglu R, Akdag-Kose A, KayabalI M, Yazganoglu KD, Disci R, Erzengin D, Azizlerli G. Vascular involvement in Behcet's disease: a retrospective analysis of 2319 cases. Int J Dermatol. 2006. 45:919–921.

27. Rohani M, Jogestrand T, Ekberg M, van der Linden J, Kallner G, Jussila R, Agewall S. Interrelation between the extent of atherosclerosis in the thoracic aorta, carotid intima-media thickness and the extent of coronary artery disease. Atherosclerosis. 2005. 179:311–316.

28. Graner M, Varpula M, Kahri J, Salonen RM, Nyyssonen K, Nieminen MS, Taskinen MR, Syvanne M. Association of carotid intima-media thickness with angiographic severity and extent of coronary artery disease. Am J Cardiol. 2006. 97:624–629.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download