Abstract
Non-small cell lung cancers (NSCLC) vary in their biologic behavior. Recurrence and tumor-related mortality may be attributable to molecular abnormalities in primary tumors. This study evaluated such immunophenotypes with regard to cell cycle regulation and proliferation, apoptosis, and angiogenesis, to determine their significance for patient outcome. Core biopsies from 219 patients with NSCLC were assembled on tissue microarrays, and the expressions of p16, p21, p27, cyclin B1, cyclin E, Ki-67, caspase-3, survivin, bcl-2, VEGF, and endostatin were evaluated by immunohistochemistry. Despite previously described prognostic relevance of some of the investigated molecules, many of those markers were not directly associated with recurrence or survival. However, there was a trend for p16 immunoreactivity to be associated with a good prognosis (57% vs. 42% in 5-yr survival) (p=0.071). bcl-2 expression was strongly correlated with a better outcome (65% vs. 45% in 5-yr survival) (p=0.029), and the hazard of death for bcl-2 positive patients was 0.42 times of that for bcl-2 negative patients (p=0.047). A multivariate analysis with Cox proportional hazards model confirmed that the lymph node status (p=0.043) and stage (p=0.003) were other independent prognostic factors. Our results suggest that p16 and bcl-2 provide prognostic information independent of the TNM stage in NSCLC.
Lung cancer is a major cause of cancer deaths worldwide (1) and has become the leading cause of cancer deaths in Korea (2). Advances in cancer treatment in the past two decades have contributed to only minimal increase in survival rates of patients with non-small cell lung cancer (NSCLC) (3). Despite apparent complete resection of the primary tumor, recurrence rates remain high and the overall 5-yr survival remains poor with <15% of patients surviving 5 yr from diagnosis (1). Some tumors, regardless of clinically favorable staging, are quite aggressive and progress to fatal disease. This implies that the TNM staging of NSCLC may be an acceptable, but not a satisfactory, classification system. Recent molecular studies have provided increased understanding of the biology of lung cancer and have identified multiple factors responsible for the modulation of tumor growth and the prognosis (4-6). Nevertheless, the essential genetic features, along with factors for prognosis, have yet to be fully understood.
Altered regulation of the cell cycle is a hallmark of human cancers (6). The cell cycle is governed by cdks. An important mechanism for regulating the cdk activity involves the cdk inhibitors, which are organized into two families based on structure and function: the Cip/Kip family (p21, p27, p57) and the INK4 family (p16, p18, p19). Cyclin E/cdk2 complex is an important regulator of entry into the S phase of the cell cycle, whereas cyclin B1/cdc2 is the classic M phase-promoting factor that drives entry into mitosis (7-10). Ki-67 proliferative index appears to be associated with survival in patients with various malignancies, but results are conflicting for NSCLC. Apoptosis or programmed cell death is a crucial mechanism of cellular homeostasis in organisms (11-14). One of the hallmark features of cancer cells is their ability to evade apoptosis. Angiogenesis is the process by which new capillary beds are formed from preexisting vessels, and is important in tumor growth (15). Vascular endothelial growth factor (VEGF) is a potent growth factor for endothelial cells (16). Tumors may activate angiogenic inhibitors such as angiostatin and endostatin, which control growth by suppressing endothelial cell proliferation and angiogenesis and by indirectly increasing apoptosis in tumor cells (17).
In the present study, we used the high-throughput tissue microarray (TMA) technology combined with immunohistochemistry (IHC) analysis (18, 19) to define the clinical significance of altered expression of cell cycle regulatory or proliferation-related, apoptotic, and angiogenic factors. Immunophenotypes were correlated with patient outcome to determine their prognostic value.
Two hundred and nineteen patients with previously untreated NSCLC were included in this study. The study protocol was reviewed and approved by the Institutional Review Board at the Catholic University St. Vincent's Hospital. All patients underwent surgical resection at the Department of Thoracic Surgery. The patients were staged at the time of their surgery following the guidelines of the American Joint Committee on Cancer Staging (20). Clinical information was obtained through a computerized retrospective database of tumor registry. Patients who died within one month after surgery were excluded from the study to avoid bias of perioperative mortality. Only patients with stages I to IIIA disease and microscopically negative resection margins were included in this study. Patients did not receive neoadjuvant chemotherapy or radiotherapy. The survival duration was determined from the date of surgery for the primary lung cancer to date of last follow-up or date of death from lung cancer. Follow-up ranged from 1.6 to 117.8 months (median 38.9 months). Eighty-seven patients (39.7%) died during follow-up, and 132 were alive at the time of this study.
Hematoxylin and eosin stained slides were reviewed for confirmation of histopathologic diagnosis and for selection of adequate specimens for analysis. Histologic typing was performed according to the histological classification of lung cancer by the World Health Organization (21). When the tumors are composed of cells resembling the mature normal cells of the tissue of origin of the neoplasm, they were considered as well differentiated, and when the tumors have primitive-appearing, unspecified cells, they were considered as poorly differentiated.
Areas of viable, representative tumor were identified and marked by a pathologist (JY) for the construction of microarrays. Three to four cores, each measuring 2.0 mm in diameter, were sampled from different areas of the tumor with a precise instrument and were arrayed on a recipient paraffin block.
For immunohistochemical staining, a sensitive peroxidase-streptavidin method, as described previously, was performed using four-µm sections of these array blocks. First sections were stained for H&E to verify histology.
The antibodies for cell cycle regulation used in this study were p16 (clone 16P07, NeoMarkers, Fremont, CA, U.S.A.), p21 (clone EA10, Zymed, San Francisco, CA, U.S.A.), p27 (NeoMarkers), cyclin E (clone 13A3, NeoMarkers), and cyclin B1 (clone GNS11, NeoMarkers). Proliferative activity was determined using anti-Ki-67 (clone 7B11, Zymed). The antibodies to detect the apoptotic factors were caspase-3 (clone 3CSP03, NeoMarkers), survivin (clone 4F7, NeoMarkers), and bcl-2 (clone bcl-2-100, Zymed). The antibodies for staining of angiogenic factors were VEGF (clone A20, Santa Cruz Biotechnology, Heidelberg, Germany), and endostatin (clone 1837-46, NeoMarkers).
Briefly, tissues cut from microarrays were deparaffinized in xylene and rehydrated in graded alcohols and water. Endogenous peroxidase was blocked by soaking in 3% H2O2 at 45℃ for 4 min. The slides were microwaved in citrate buffer (2.1 g/L, pH 6.0) at 120℃ for 15 min to unmask the antigen, and treated with a protein-blocking reagent before incubation at 4℃ overnight with primary antibodies at a 1:50 dilution, as recommended by the supplier. After extensive washing, the sections were incubated at room temperature for 10 min with biotinylated anti-mouse immunoglobulin antibodies (Zymed, San Francisco, CA) at a 1:20 dilution and subsequently with streptavidin-biotin peroxidase complexes at a 1:25 dilution. Reaction products were visualized by immersing slides in 3,3'-diaminobenzidine tetrahydrochloride. Counterstaining was performed with hematoxylin. All series included positive and negative controls. Negative controls were prepared by omitting the primary antibodies and known positive controls were included in each run.
Three investigators independently evaluated the results from the immunohistochemical staining in a blinded fashion and obtained the identical results in 92% of the cases. For the discordant samples, slides were reviewed jointly and a consensus was reached. For the evaluation of immunoreactivity in tumor cells, a dichotomised scoring system was used as follows: p16 positivity if >80% of tumor cells demonstrated either nuclear or cytoplasmic staining; p21 positivity if >5% tumor nuclei stained; p27 positivity if >20% of tumor nuclei stained; high Ki-67 labeling index and high cyclin E labeling index if >30% tumor cells stained; caspase-3 positivity if nuclear or cytoplasmic staining was present in >25% of tumor cells; survivin or bcl-2 positivity if >10% tumor cell stained; VEGF expression if >5% of tumor cells stained; endostatin positivity if scores between 3 and 6 (the sum of the percentage of tumor cells [0, 1=<25%, 2=26-50%, 3=>50%] and the staining intensity [1-3]) (9, 12, 18, 22-26).
Statistical analysis was carried out using a software package (SPSS 13.0, Seoul, Korea). Survival probabilities were estimated using the Kaplan-Meier method and then compared with the log rank test. The influence of various clinicopathologic variables on the survival was assessed with the Cox proportional hazards model. The level of significance was set at p<0.05.
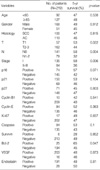
Complete clinical and histologic data were available for all patients. One hundred and sixty-eight patients (76.7%) were male and the mean age was 65.8 yr (SD 9.9; median 67; age range 19-89). Patient characteristics are shown in Table 1.
A significant variation was present in the expression of the individual immunohistochemical markers in tumor tissues. The highest rate of positivity was noted for VEGF in 92.7% of the tumors. The lowest rate of expression was observed with survivin in 2.7% of the samples. The expression profiles of all markers are presented in Table 2.
Of the cell-cycle regulation and proliferation-related proteins, p16 was expressed in 74 of 219 (33.8%) cases (Fig. 1A). There were significant differences in T status and clinical stage between the p16-positive and -negative patients (p=0.049 and 0.038, respectively); normal p16 expression was more frequent in patients with T1 than in those with T2-3 (36.8% vs. 32.7%), and in patients with stage I than in patients with stage II-III (42.2% vs. 20.2%). Seventy-four tumors (68.5%) expressed p21. p21 expression was correlated with regional lymph node status (p=0.037) and with clinical stage (p=0.012). p27 immunoreactivity was identified in 71 cases (32.4%) and was more common in adenocarcinomas than in squamous cell carcinomas (p=0.035). Cyclin-B1 immunostaining was detected in 10 tumors (4.6%) without a clinically significant pattern. Cyclin E was positive in 84 samples (38.4%). In adenocarcinomas, cyclin-E staining was associated with differentiation (p=0.001); 3 of 29 well-differentiated tumors (10.3%), 25 of 64 moderately differentiated tumors (39.1%), and 13 of 26 poorly differentiated tumors (50%) were positive for cyclin-E (data not shown). For Ki-67, a high Ki-67 proliferative index was observed in 17 of 219 patients (7.8%).
Of the apoptotic factors analyzed in the study, caspase-3 was expressed in 84 tumors (38.4%). Reactivity for caspase-3 was present in 46 of 141 node-negative patients (32.6%) and 38 of 78 node-positive patients (48.7%) (p=0.049). Survivin expression was rarely seen, with a mere positive staining rate of 2.7%. bcl-2 immunoreactivity was observed in 25 cases (11.4%) (Fig. 1B).
Of 219 patients, 203 (92.7%) and 191 (87.2%) had positive immunohistochemical staining for VEGF and endostatin, respectively. There was no significant influence of these two factors on histology, T status, regional lymph node status and clinical stage.
Five-year survival rates according to clinicopathologic variables and immunohistochemical expression profiles are summarized in Table 3. T status (53% vs. 44%; p=0.022), regional lymph node status (58% vs. 32%; p=0.004) and clinical stage (58% vs. 36%; p=0.006) demonstrated a significant correlation with 5-yr survival, respectively, based on the log-rank test.
Of 11 markers analyzed, p16 and bcl-2 had an impact on the 5-yr survival. There was a trend for p16 immunoreactivity to be associated with a good prognosis (5-yr survival, 57% vs. 42%; p=0.071). Patients with negative or positive bcl-2 expression had 5-yr survival rates of 45% and 65%, respectively, which was statistically significant (p=0.047, Mantel-Cox log-rank test) (Fig. 2).
Combined analysis of bcl-2 and p16 expression identified 5-yr survival rates of 73.3% and 38.6% in patients with at least one of these factors and those with neither of these factors, respectively. These molecular phenotypes had a significant influence over clinical outcome (p=0.002). In addition, Hazard ratio was used to estimate hazard of death due to NSCLC for the baseline variables (Table 4). The hazard of death for node-positive patients was 1.855 times of the hazard of death for node-negative patients (p=0.004). The hazard of death for patients with tumor stages II-III was 1.994 times of the hazard of death for patients with tumor stage I (p=0.002). For the effects on survival, bcl-2 expression was statistically significant with a p-value of 0.029. The multivariate analysis for overall survival revealed three independent prognostic factors: regional lymph node status, stage and bcl-2 expression. Regional lymph node status (N1-2 vs. N0) was a significant and independent unfavorable prognostic factor (p=0.043), as was the advanced clinical stage (II-III vs. I) (p=0.003), whereas immunohistochemical expression of bcl-2 (positive vs. negative) was a significant and independent favorable prognostic factor (p=0.047).
Management of a patient with NSCLC could be determined by the aggressiveness of the cancer, and the aggressive cancer phenotypes are characterized by a number of molecular abnormalities. Because of the complexity of the molecular biology of NSCLC, studies on a single molecular factor have not yet been successful in creating biological risk assessment. A few studies support the possibility that multiple markers might be more informative than any single marker for the prediction of clinical outcome of NSCLC (4, 8). This has prompted us to investigate 11 biologic markers, including those involved in cell cycle control and proliferation, apoptosis and angiogenesis, and to construct a multivariate analysis for these factors predicting the patients' prognosis.
The major findings of our study are as follows: 1) expression of p16 was associated with T status in NSCLC; 2) expression of p21 or caspase-3 correlated with regional lymph node status; 3) expression of p16 or p21 correlated with stage; 4) patients with positive bcl-2 immunostaining or p16 immunoreactivity or both had a higher 5-yr survival rate; 5) bcl-2 expression constitutes an independent prognostic parameter, along with regional lymph node status and stage.
Cyclin E is involved in the regulation of G1-S transition of the cell cycle, whereas cyclin B1 is involved in the regulation of G2-M transition. An important mechanism for regulating the CDK activity involves the CDK inhibitors, including p21, p27, p57, p16, p18 and p19. Some of the alterations in expression of these proteins, which lead to the failure of cell cycle arrest, have been demonstrated to be predictive for outcome and thus may serve as markers of more malignant phenotypes (27). In the present study, p16 and p21 were found to correlate with T, N or stage. Only p16 had a direct impact on the 5-yr survival in patients with NSCLC. This is in agreement with the results of the study by Esposito et al. (8). In their multivariate analysis on cell cycle regulator proteins, the only immunohistochemical parameter that influenced overall survival was p16. It is suggested that cell cycle regulatory proteins may have different and multifunctional properties on cell proliferation and may contribute to their different effects on survival. The p16 expression may have a great value in identifying NSCLC patients with better prognosis.
As bcl-2 inhibits apoptosis, the bcl-2 overexpression should theoretically favor the malignant process and result in a poor outcome. However, there have been several studies describing a favorable outcome in NSCLC expressing bcl-2, although others have shown no survival advantage or even a worse prognosis (14, 26-29). Poleri et al. (26) described a poor outcome in patients with positive bcl-2 tumor. Cox et al. (14) investigated 167 patients with resected stage I-IIIA NSCLC and found a trend for bcl-2 expression to be associated with an improved survival, which was consistent with our findings. The reasons for this association are poorly understood. These conflicting results may have been due to the difference in the nature of tumor samples analyzed. In contrast to Poleri et al's. study limited to the stage I NSCLC (26), we included stage I-IIIA tumors, as in Cox et al. (14). The criteria for interpreting the results as positive or negative may also have attributed to this difference. Cytoplasmic bcl-2 staining was considered either positive or negative according to the investigators using different percentage criteria. While only samples with at least 20% of the tumor were regarded as being positive, immunoreaction in more than 5% or even 1% of the tumor cells was also considered as being expressed by others (14, 30, 31). Further investigations using the identical scoring criteria are necessary to establish a significance of bcl-2 expression in lung cancer outcome.
The bcl-2 expression has been demonstrated to be inversely related with angiogenesis, a finding that may account for the favorable prognosis. The present study, however, revealed a significant association between the bcl-2 immunoreactivity and VEGF (data not shown), suggesting that other mechanisms independent of the angiogenesis pathway may account for a better survival in patients with bcl-2 positivity. It has been suggested that loss of bcl-2 expression may represent tumor de-differentiation (14, 32).
VEGF is a potent growth factor for endothelial cells. While angiogenesis and increased VEGF have been shown to adversely affect NSCLC outcome by some authors, other studies showed no prognostic value of VEGF (27). We found no correlation between VEGF immunoreactivity and prognosis. Endostatin, one of angiogenic inhibitors, controls growth by suppressing endothelial cell proliferation and angiogenesis and by indirectly increasing apoptosis in tumor cells. The expression of endostatin has been demonstrated in several solid malignancies, including rectal cancer, ovarian cancer, hepatocellular carcinoma and NSCLC (17, 27, 33). Although higher expression of endostatin would be expected to improve survival, the impact of endostatin expression on clinical outcome remains unclear. Iizasa et al. (17) reported that the expression of collagen XVIII, an endostatin precursor, in tumor tissue correlated with elevated levels of circulating serum endostatin and was strongly associated with a poorer outcome in NSCLC. In this study, the majority of NSCLC cases demonstrated endostatin immunoreactivity. However, no statistically significant effect on survival was found.
In conclusion, the present study demonstrated that NSCLCs have heterogeneous expression of cell cycle regulatory proteins, apoptotic factors, and angiogenic factors. However, the expressions of p16 and bcl-2 were associated with a trend toward a better survival in this selected group of patients. These findings warrant additional molecular and clinicopathologic studies of those markers and their related pathways potentially relevant to prognosis in NSCLC.
Figures and Tables
Fig. 2
Kaplan-Meier survival plots for non-small cell lung carcinoma. (A) p16 positive (solid line) vs. negative (dotted line); (B) bcl-2 positive (solid line) vs. negative (dotted line). p-values by Mantel-Cox log-rank test were 0.071 (A) and 0.047 (B).

References
1. Cox G, Jones JL, Andi A, Waller DA, O'Byrne KJ. A biological staging model for operable non-small cell lung cancer. Thorax. 2001. 56:561–566.

2. Korean National Statistical Office. Annual report on the cause of death statistics. 2001.
3. Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001. 93:277–283.

4. Kwiatkowski DJ, Harpole DH Jr, Goleski J, Herndon JE II, Dar-Bin S, Richards W, Blanco R, Xu H, Strauss G, Sugarbaker DJ. Molecular pathologic substaging in 244 stage I non-small-cell lung cancer patients: clinical implications. J Clin Oncol. 1998. 16:2468–2477.

5. Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000. 287:1969–1973.

6. Sherr CJ. Cancer cell cycle. Science. 1996. 274:1672–1677.
7. Cordon-Cardo C. Mutation of cell cycle regulators. Biological and clinical implications of human neoplasia. Am J Pathol. 1995. 147:858–911.
8. Esposito V, Baldi A, Tonini G, Vincenzi B, Santini M, Ambrogi V, Mineo TC, Persichetti P, Liuzzi G, Montesarchio V, Wolner E, Baldi F, Groeger AM. Analysis of cell cycle regulator proteins in non-small cell lung cancer. J Clin Pathol. 2004. 57:58–63.
9. Dosaka-Akita H, Hommura F, Mishina T, Ogura S, Shimizu M, Katoh H, Kawakami Y. A risk-stratification model of non-small cell lung cancers using cyclin E, Ki-67, and ras -21: Different roles of G1 cyclins in cell proliferation and prognosis. Cancer Res. 2001. 61:2500–2504.
10. Volm M, Koomagi R, Mattern J, Efferth T. Expression profiles of genes in non-small cell lung carcinomas from long-term surviving patients. Clin Cancer Res. 2002. 8:1843–1848.
11. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.

12. Koomagi R, Volm M. Relationship between the expression of Caspase-3 and the clinical outcome of patients with non-small cell lung cancer. Anticancer Res. 2000. 20:493–496.
13. Falleni M, Pellegrini C, Marchetti A, Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G, Bosari S. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol. 2003. 200:620–626.

14. Cox G, Jones JL, Andi A, Abrams KR, O'Byrne KJ. Bcl-2 is an independent prognostic factor and adds to a biological model for predicting outcome in operable non-small cell lung cancer. Lung Cancer. 2001. 34:417–426.

15. Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995. 333:1757–1763.

16. Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1.KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999. 188:369–377.

17. Iizasa T, Chang H, Suzuki M, Otsuji M, Yokoi S, Chiyo M, Motohashi S, Yasufuku K, Sekine Y, Iyoda A, Shibuya K, Hiroshima K, Fujisawa T. Overexpression of collagen XVIII is associated with poor outcome and elevated levels of circulating serum endostatin in non-small cell lung cancer. Clin Cancer Res. 2004. 10:5361–5366.

18. Hoos A, Nissan A, Stojadinovic A, Shia J, Hedvat CV, Leung D, Paty P, Klimstra D, Cordon-Cardo C, Wong WD. Tissue microarray molecular profiling of early, node-negative adenocarcinoma of the rectum: a comprehensive analysis. Clin Cancer Res. 2002. 8:3841–3849.
19. Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch M, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nature Med. 1998. 4:844–847.

20. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997. 111:1710–1717.

21. WHO. Histological classification of tumors of the lung. 2004. Lyon;10.
22. Oshita F, Ito H, Ikehara M, Ohgane N, Hamanaka N, Nakayama H, Saito H, Yamada K, Noda K, Mitsuda A, Kameda Y. Prognostic impact of surviving, cyclin D1, integrin beta 1, and VEGF in patients with small adenocarcinoma of stage I lung cancer. Am J Clin Oncol. 2004. 27:425–428.
23. Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of cyclin B1 and Wee 1 expression in non-small cell lung cancer. Ann Oncol. 2004. 15:252–256.
24. Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of surviving and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000. 6:127–134.
25. Rodel C, Grabenbauer GG, Rodel F, Birkenhake S, Kuhn R, Martus P, Zorcher T, Fursich D, Papadopoulos T, Dunst J, Schrott KM, Sauer R. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000. 15:1213–1221.
26. Poleri C, Morero JL, Nieva B, Vazquez MF, Rodriguez C, de Titto E, Rosenberg M. Risk of recurrence in patients with surgically resected stage I non-small cell lung carcinoma: Histopathologic and immunohistochemical analysis. Chest. 2003. 123:1858–1867.
27. Singhal S, Vachani A, Antin-Ozerkis D, Kaiser LR, Albelda SM. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res. 2005. 11:3974–3986.

28. Silvestrini R, Costa A, Lequaglie C, Mochen C, Veneroni S, Leutner M, Ravasi G. bcl-2 protein and prognosis in patients with potentially curable non-small -cell lung cancer. Virchows Arch. 1998. 432:441–444.
29. Ohsaki Y, Toyoshima E, Fujiuchi S, Matsui H, Hirata S, Miyokawa N, Kubo Y, Kikuchi K. bcl-2 and p53 protein expression in non-small cell lung cancers: correlation with survival time. Clin Cancer Res. 1996. 2:915–920.
30. Ritter JH, Dresler CM, Wick MR. Expression of bcl-2 protein in stage T1N0M0 non-small cell lung carcinoma. Human Pathol. 1995. 26:1227–1232.
31. O'Neill AJ, Staunton MJ, Gaffney EF. Apoptosis occurs independently of bcl-2 and p53 over-expression in non-small cell lung carcinoma. Histopathology. 1996. 29:45–50.
32. Koukourakis MI, Giatromanolaki A, O'Byrne KJ, Whitehouse RM, Talbot DC, Gatter KC, Harris AL. Potential role of bcl-2 as a suppressor of tumor angiogenesis in non-small cell lung cancer. Int J Cancer. 1997. 74:565–570.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download