Abstract
Although mycobacterial culture and the subsequent drug-susceptibility test (DST) for anti-tuberculosis (TB) drugs take several months to complete using solid media, there are no reports on the turnaround times of these tests under clinical conditions. The aim of this study was to determine the interval between initiation of anti-TB treatment and receipt of DST requested at an outpatient clinic. We prospectively enrolled patients with culture-positive pulmonary TB at Seoul National University Hospital from September 2002 to December 2004. Patients were followed up monthly. Mycobacterial cultures were done using Ogawa media at Seoul National University Hospital. DST were performed at the Korean Institute of Tuberculosis. Of the 104 patients enrolled, 54 were male. The median age was 41 yr. The median interval from initiation of anti-TB treatment to receipt of mycobacterial culture results by clinicians was 37 days (range, 0-89 days). The median interval from initiation of treatment to confirmation of DST by requesting clinicians was 80.5 days (range, 28-145 days). Clinicians only received the results of DST more than two months after initiation of treatment when they followed up patients monthly and mycobacterial culture was performed using solid media.
The drug-susceptibility test (DST) is essential for detecting drug-resistant tuberculosis (TB) and designing effective regimens for treating individual patients. Although the clinical accuracy of DST has been debated, it produces quite reliable results for susceptibility to isoniazid, rifampicin, and streptomycin (1). However, the proportional method using Löwenstein-Jensen media usually takes longer than four weeks to execute (2, 3).
Although recent guidelines recommend that pyrazinamide be withdrawn after two months of intensive treatment (4-6), the long turnaround time of the DST based on conventional Löwenstein-Jensen media could prolong the use of pyrazinamide because clinicians may wait for confirmation of drug resistance before terminating treatment.
However, there are no reports on the actual turnaround times of DSTs for which solid media are used when TB patients are treated in outpatient clinics. The aim of this study is to determine the interval between initiation of anti-TB treatment and receipt of the results of solid-media DSTs for patients with culture-positive pulmonary TB who were treated in an outpatient clinic.
We prospectively enrolled patients for whom anti-TB medication was started at Seoul National University Hospital, a tertiary referral hospital, from September 2002 to December 2004. Patients who agreed to participate in the study were followed up on a monthly basis, after their first revisit two weeks from initiation of anti-TB treatment. Of these, only patients with culture-confirmed TB were included in the analysis. We excluded patients who missed a scheduled visit and failed to present themselves at the clinic during the subsequent fortnight or who were transferred out before the results of their DSTs were received by their clinicians. However, patients who, for various reasons, visited the clinic before the scheduled follow-up day were included in the analysis. Treatment consisted of daily self-administered therapy. Direct observation of ingestion of anti-TB drugs is not included in the national TB control policy of Korea (7). The protocol had been approved by Seoul National University Hospital ethics review committee and the written consents were obtained from all participants.
Mycobacterial cultures were done in 3% Ogawa media at Seoul National University Hospital. Experienced technicians recorded the results of cultures every monday and designated negative if colonies were absent after nine weeks of incubation. Colonies from positive cultures were sent on tuesdays for DSTs to the Korean Institute of Tuberculosis, the supranational reference laboratory for mycobacterial culture and DSTs. The transfer time from Seoul National University Hospital to the Korean Institute of Tuberculosis was about two hours. DSTs for anti-TB drugs were performed using Lowenstein-Jensen media and the proportional method.
The results of DSTs were sent from the Korean Institute of Tuberculosis to Seoul National University Hospital on tuesdays. Clinicians checked whether reports had been received each time their patients visited the clinic. If a report was not received by the time of the visit, the duty clinician contacted the Korean Institute of Tuberculosis through telephone or via their website, http://tb.knta.or.kr/, to confirm that the results were unavailable.
Of the 167 patients who participated, 119 were diagnosed with culture-positive pulmonary TB and were enrolled in the study. Of the 119, 15 were excluded from the analysis because they missed a scheduled visit and failed to present themselves at the clinic during the subsequent fortnight or were transferred out before their clinicians received the results of their DSTs. Data from 104 patients were included in the final analysis. Of the patients, 54 (55%) were male. The median age was 41 yr (range 17-85 yr). Forty-six patients (44%) were diagnosed as having both smear-positive and culture-positive pulmonary TB, and the remainder had smear-negative but culture-positive TB (Table 1).
The median interval between initiation of anti-TB treatment and receipt of reports of positive mycobacterial cultures by Seoul National University Hospital was 20 days (range, 0-71 days). However, receipt of culture results by clinicians was delayed (median, 37 days; range, 0-89 days). The median interval between a request for a DST and receipt of the report was 28.5 days (range, 14-88 days). From the date of initiation of anti-TB treatment, 67 days (range, 25-124 days) elapsed until Seoul National University Hospital received the DST results, and 80.5 days (range, 28-145 days) elapsed until the requesting clinicians received them (Fig. 1).
By the end of second month of anti-TB treatment, DST results for only 16 patients (15%) had been received by clinicians. By the third month of treatment, DST results of 57 patients (55%) had been received. The cumulative percentages of DST confirmation at 4, 5, and 6 months were 89%, 99%, and 100%, respectively (Fig. 2). Distribution of patients according to their intervals of mycobacterial culture and DST was summarized in Table 2.
Current guidelines for treatment of TB published by the American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America and the British Thoracic Society recommend performing DSTs whenever possible (5, 6). In addition, these guidelines suggest that pyrazinamide be discontinued after confirming DST results excluding drug-resistant TB, although the guidelines of the Korean Society of Tuberculosis and Respiratory Diseases recommend that pyrazinamide be discontinued after two months of the intensive treatment phase regardless of whether DST results are available or not (8). The long turnaround times of DSTs conducted using solid media could prolong the two-month intensive treatment phase (2, 3). However, no reports have been published on the turnaround times of solid-media DSTs in clinical settings.
Our study showed that the median interval from initiation of anti-TB treatment to receipt of DST results by requesting clinicians was 80.5 days (range, 28-145 days). In addition, the results of DSTs for which Lowenstein-Jensen media were used were available to clinicians by the completion of two months of the intensive phase in only 15% of cases when follow-up was conducted on a monthly basis. This indicates that the duration of the intensive treatment phase would exceed two months in 85% of TB patients if pyrazinamide treatment were discontinued only after DST results were received.
For the treatment of isoniazid-resistant TB, prolongation of the continuation phase of treatment without pyrazinamide is recommended if DST results are available later than the second month of the intensive phase of treatment (5, 6). However, the development of resistance to rifampicin in a patient who had isoniazid-resistant TB and had been given prolonged rifampicin and ethambutol treatment was recently reported (9, 10). Although prolonged use of pyrazinamide is unnecessary for most TB patients, it is best to know the results of DSTs within two months of initiation of anti-TB treatment. A shorter DST turnaround time of 28 days could be achieved by using broth-based systems such as BACTEC (11-13), MB/BacT (14), ESP (15), or MGIT (16, 17). However, the cost of broth-based systems is too high for general use, except in a few developed countries. In addition, DSTs for resistance to second-line anti-TB drugs still have to be performed using solid media.
Considering that a median of 20 days (range, 0-71 days) elapsed between initiation of treatment and receipt of confirmation of a positive mycobacterial culture, and that a median of 28.5 days (range 14-88 days) elapsed between delivery of samples for DSTs and receipt of results, the interval between initiation of treatment and receipt of DST results might be reduced by improvement of the process involved in requesting or reporting DSTs.
For example, a system of automatically requesting DST with mycobacterial colonies from every patient who has not had a previous DST might reduce the interval to receipt of DST results, median 14 days in our study. In addition, direct report of DST results from Korean Institute of Tuberculosis to the physicians could save another 14.5 days. This direct reporting system could be easily achieved through e-mail or mobile phone. However, the interval could not be reduced to less than eight weeks after initiation of treatment for all patients because of the wide range of periods required to complete mycobacterial cultures and DSTs using solid media. In this context, cost-effectiveness of the introduction of liquid media in Korea, an intermediate TB burden country, should be analyzed through future studies. In addition, the detection of resistance using molecular technique could be considered.
In conclusion, clinicians only received the results of DST more than two months after initiation of treatment when they followed up patients monthly and the mycobacterial culture was performed using solid media.
Figures and Tables
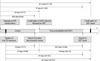 | Fig. 1Intervals related to the transmission of the results of drug-susceptibility tests. All numbers in this figure represent days. *When anti-TB treatment was started after receipt of the result of positive mycobacterial culture, the interval was regarded as 0 days. †Drug-susceptibility test. |
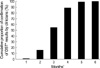 | Fig. 2Monthly receipt of the results of drug-susceptibility tests after initiation of the anti-TB treatment by clinicians *Drug-susceptibility test, †Months mean duration of anti-tuberculosis treatment. |
References
1. Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005. 25:564–569.

2. Muralidhar S, Srivastava L. Evaluation of three methods to determine the antimicrobial susceptibility of Mycobacterium tuberculosis. Indian J Med Res. 2004. 120:463–467.
3. Schaberg T, Reichert B, Schulin T, Lode H, Mauch H. Rapid drug susceptibility testing of Mycobacterium tuberculosis using conventional solid media. Eur Respir J. 1995. 8:1688–1693.
4. WHO. Treatment of tuberculosis; Guidelines for National Programmes 2003.
5. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.
6. British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 1998. 53:536–548.
7. Seung KJ, Bai GH, Kim SJ, Lew WJ, Park SK, Kim JY. The treatment of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2003. 7:912–919.
8. Korean Society of Tuberculosis and Respiratory Diseases. Guidelines for the diagnosis and treatment of tuberculosis 2005.
9. Seung KJ, Gelmanova IE, Peremitin GG, Golubchikova VT, Pavlova VE, Sirotkina OB, Yanova GV, Strelis AK. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis. 2004. 39:1321–1328.

10. Koh WJ, Kwon OJ, Park YK, Lew WJ, Bai GH. Development of multidrug resistance during treatment of isoniazid-resistant tuberculosis. Eur Respir J. 2005. 26:557.

11. Anargyros P, Astill DS, Lim IS. Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J Clin Microbiol. 1990. 28:1288–1291.

12. Steadham JE, Stall SK, Simmank JL. Use of the BACTEC system for drug susceptibility testing of Mycobacterium tuberculosis, M. kansasii, and M. avium complex. Diagn Microbiol Infect Dis. 1985. 3:33–40.

13. Roberts GD, Goodman NL, Heifets L, Larsh HW, Lindner TH, McClatchy JK, McGinnis MR, Siddiqi SH, Wright P. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983. 18:689–696.

14. Tortoli E, Mattei R, Savarino A, Bartolini L, Beer J. Comparison of Mycobacterium tuberculosis susceptibility testing performed with BACTEC 460TB (Becton Dickinson) and MB/BacT (Organon Teknika) systems. Diagn Microbiol Infect Dis. 2000. 38:83–86.

15. Ruiz P, Zerolo FJ, Casal MJ. Comparison of susceptibility testing of Mycobacterium tuberculosis using the ESP culture system II with that using the BACTEC method. J Clin Microbiol. 2000. 38:4663–4664.

16. Tortoli E, Benedetti M, Fontanelli A, Simonetti MT. Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: comparison with the radiometric BACTEC 460TB method and the agar plate method of proportion. J Clin Microbiol. 2002. 40:607–610.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download