INTRODUCTION
Over the past few years, there has been recognition of a distinctive group of renal tumors composed grossly of cystic and solid portion and microscopically of stromal and epithelial proliferation. While it is not clear whether they represent a heterogenous group or a single disease entitiy, these tumors have been reported under various names such as adult mesoblastic nephroma, cystic nephroma, mixed epithelial and stromal tumor and some others. We believe that the descriptive and unifying term of mixed epithelial and stromal tumor is appropriate for this group of tumors until further characterization is made and report a case of renal tumor which appears to represent a good example of this entity.
CASE REPORT
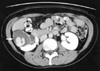
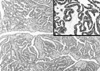
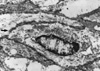
A 47-yr-old woman was detected to harbor a right renal mass by ultrasonography on routine examination. Microscopic hematuria was also present at that time but there were no other abnormal clinical or laboratory findings or past history of medical problem. On computed tomography, the mass measured 7 cm in the largest dimension and was multiseptated with irregularly enhancing solid component (Fig. 1). Radical nephrectomy was performed under the diagnosis of renal cell carcinoma. The mass was occupying mid to lower pole of the right kidney close to renal sinus and partially protruding into renal pelvis. It measured 8×6 cm and was largely cystic with a whitish yellow solid mural nodule of 3×3 cm (Fig. 2) and there was no involvement of ureter or blood vessels. Microcystic lesions were present with regional sponge-like appearance and focal calcification was also found. Upper half of renal parenchyma uninvolved by the mass was grossly unremarkable. Microscopically, solid portion was composed of irregularly arranged bundles of cigar-shaped spindle cells sprinkled with a few inflammatory cells in the fibrillar background (Fig. 3). Cellular atypia was negligible and mitosis was not encountered. The nodule of spindle cells displayed hypocelluar areas at the periphery entrapping several tubular structures which occasionally exhibited cyst-like dilatation. Epithelial proliferation was exuberant around the sponge-like areas and occupied an area measuring approximately 1.5 cm in largest dimension. Eosinophilic columnar epithelial cells containing occasional single unconspicuous nucleolus constituted papillary structures with fibrovascular core and intervening foamy histiocytes (Fig. 4). Immunohistochemically, the spindle cells were positive for vimentin, smooth muscle actin, but negative for CD34, desmin, HMB45, estrogen receptor and progesterone receptor while epithelial cells were reactive for epithelial membrane antigen, high molecular weight cytokeratin and pancytokeratin. On electron microscopic examination, the spindle cells had many subplasmalemmal pinocytotic vesicles and intracytoplasmic filaments occasionally forming dense bodies, thus suggesting myofibroblastic differentiation (Fig. 5).
DISCUSSION
Michal and Syrucek first proposed the term of mixed epithelial and stromal tumor of the kidney in 1998 (1) and later Adsay et al. reported a group of 12 patients under the same name and regarded the diagnosis as an appropriate preliminary title for that category of tumor which is characterized grossly by a mixture of solid and cystic areas and is microscopically composed of proliferations of stromal and epithelial cells (2). Various diagnoses have been rendered to tumors with similar morphologic findings such as adult mesoblastic nephroma (3-6), cystic hamartoma of pelvis (7), cystic nephroma (8) or mature nephroblastic tumor and cystic partially differentiated nephroblastoma (9). It remains to be determined whether these tumors are comprised of a heterogenous group of tumors or represent one distinct entity. However, these tumors basically share similar clinicopathologic characteristics and therefore it appears reasonable, at least for the present, to regard them as a single entity.
Approximately 50 cases of such tumors have been reported in the literature (2-5) and three cases have been documented in Korean literature under the name of adult mesoblastic nephroma (10-12). Common clinical presentations were those of usual renal mass lesions such as flank pain, hematuria, symptoms of infection or no attributable symptoms. Mean tumor size is approximately 6 cm and the tumor shows a female preponderance centered around perimenopausal age and frequent reactivity for estrogen or progesterone receptors. And in rare reported cases of male patients, there was a history of hormone therapy (2). These facts led to the suggestion of hormonal mechanism in pathogenesis and the estrogen- and progesterone-receptor positivity are sometimes deemed sufficient characteristics to warrant its utility as an adjunct diagnostic criteria for this tumor. However, our case lacked evidence of hormonal receptor expression and therefore it is reasonable to conclude that not all cases of mixed epithelial and stromal tumor implicate hormonal mechanism in pathogenesis and it also can be speculated that this presumed difference in pathogenesis might eventually result in subclassification of this tumor after accumulation of further data.
The stromal component of mixed epithelial and stromal tumor varies in cellularity and can assume various histologic appearance reminiscent of leiomyoma, solitary fibrous tumor or ovarian stroma. Ovarian stroma, frequently found in mixed epithelial and stromal tumor, is absent in this case, which might be related to estrogen and progesterone receptor negativity of this case.
Epithelial proliferation in this case is exuberant and spans an area over 1 cm in diameter. This feature is worrisome especially when we consider the newly accepted criteria of 0.5 cm as a cut-off point between papillary adenoma and carcinoma (13). However, malignancy of epithelial component is not yet documented in mixed epithelial and stromal tumor whereas there is a report of stromal malignancy (14). Therefore, while it appears reasonable to accept, at least for the present, that benign epithelial proliferation can be florid, the possibility cannot be completely ruled out that there might develop epithelial malignancy.
Adult mesoblastic nephroma was first reported in 1973 (15) and was the primary diagnosis in a majority of this group of tumors (3-6). While it is clear that this tumor shares a few morphologic characteristics of congenital mesoblastic nephroma, it also appears evident that there are a few unmistakable differences. Firstly and most importantly, the epithelial proliferation seems an integral neoplastic component in this tumor (2) whereas in congenital mesoblastic nephroma, it is more likely entrapped epithelial portion with occasional metaplastic changes and focal hyperplasia. Furthermore, genetic alterations typical of congential mesoblastic nephroma were not found in this tumor (16). And frequent infiltration into surrounding renal parenchyma found in congenital mesoblastic nephroma is rarely encountered (4). Therefore this tumor is more likely unrelated to mesoblastic nephroma and it appears more reasonable to apply the descriptive term of mixed epithelial and stromal tumor at least for the present under which name it is included in the current WHO classification (13).
When we accept the entity of mixed epithelial and stromal tumor, the differential diagnosis is limited. The spindle cell component of mixed epithelial and stromal tumor can be found in solitary fibrous tumor (17) or angiomyolipoma (18), both of which can be excluded with immunostaining and by lack of epithelial proliferation. Biphasic renal lesions may encompass tumors such as nephroblastoma, cystic partially differentiated nephromablastoma and cystic embryonal sarcoma (19), all of which are rare in adulthood or contain malignant mesenchymal component. Rare benign biphasic renal tumor such as nephrogenic adenofibroma (20) can be differentiated on the basis of negative immunoreactivity to actin or desmin and the lack of cystic component.
In summary, we report a case of renal tumor showing unique histologic features of biphasic epithelial and stromal component and briefly discuss the differential diagnosis and its relationship with mesoblastic nephroma.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download