Abstract
Soft tissue uptake of Tc-99m labeled bone seeking agents, such as Tc-99m 3,3-diphosphono-1,2-propanedicarboxylic acid (DPD), is commonly seen in clinical practice, even though bone scintigraphy is mainly used to detect bone disease. However, gastric uptake of bone agents in patients with gastric cancer is very rare. And it has been reported that calcified gastric adenocarcinoma appears in only about 5% of all gastric cancer. We report a rare case of bone scintigraphy, single photon emission computed tomography and computed tomography fusion images that demonstrated diffuse gastric uptake of Tc-99m DPD in a patient with advanced gastric cancer.
Calcifications within primary tumors can be seen in thyroid, breast, and ovarian neoplasms. They are also described within benign lesions of the gastrointestinal tract, such as leiomyomas and angiomas, or in metastases from colonic carcinoma. However, calcifications within primary gastric cancer are a rare finding (1, 2). Moreover, gastric uptake of bone seeking agents in a patient with advanced gastric cancer is an even rare entity.
Tc-99m 3,3-diphosphono-1,2-propanedicarboxylic acid (DPD) bone scintigraphy is used primarily for skeletal imaging to detect abnormalities of the bone. However, a variety of soft tissue abnormalities can also be detected on bone scintigraphy through accumulation of bone seeking agents. Neoplastic, hormonal, inflammatory, ischemic, traumatic, excretory, and artifactual entities demonstrate abnormal soft-tissue uptake of Tc-99m DPD (3).
Recently, we experienced a case of bone scintigraphy that demonstrated diffusely increased gastric uptake of Tc-99m DPD in a patient with advanced gastric cancer. We report this case along with a review of the literatures.
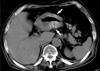
A 69 yr-old male patient was admitted to our hospital due to epigastric discomfort for 2 months duration. Epigastric discomfort was exacerbated after meals. An upper GI endoscopy revealed a huge ulcerative creator on the posterior wall of the prepyloric area with creamy exudates and a biopsy diagnosed the lesion to be adenocarcinoma. Non-contrast enhanced computed tomography (CT) of the abdomen showed a mass involving the lower body and antrum of the stomach with diffuse and circumferential wall thickening, punctate calcifications within the gastric walls, and several lymphadenopathies along the retropancreatic area (Fig. 1). Laboratory studies revealed normochromic and normocytic anemia (hemoglobin of 10.3 g/dL, normal; 12-16 g/dL), but other laboratory findings including serum calcium (normal; 8.4-10.2 mg/dL) and phosphorus (normal; 2.5-4.5 mg/dL) concentrations were normal.
To evaluate the extent of the disease, 3 hr after injection of 20 mCi (740 MBq) of Tc-99m DPD, bone scintigraphy and single photon emission computed tomography (SPECT) were performed using a dual-head gamma camera (Vertex; ADAC laboratories, Milpitas, CA, U.S.A.). Bone scintigraphy showed gastric uptake but no evidence of bone metastasis (Fig. 2). The gastric uptake was thought to be calcified tumoral uptake. To confirm this, fusion images were constructed by combining morphologic CT and functional SPECT images on a computer workstation. The SPECT and CT fusion images show the intense Tc-99m DPD uptake to be located in the anterior and posterior wall of the gastric antrum (Fig. 3).
The first report of calcified gastric cancer was by Gruber in 1913 (4), and subsequently several other cases were reported (5, 6). When histology was considered, most of these tumors were proved to be mucinous adenocarcinomas. It has been reported that mucinous gastric adenocarcinoma compromise about 5% of all gastric cancers (7). To explain the presence of calcifications, "dystrophic" and "metastatic" mechanisms have been proposed (8). Dystrophic calcifications develop in necrotic tissues, probably because denatured proteins preferentially bind phosphate ions, which react with calcium ions to form a precipitate of calcium phosphate. In these cases plasma levels of calcium are within normal range. Metastatic calcifications are simple mineral deposits in undamaged normal tissues caused by persistent hypercalcemia. In our case, normal level of serum calcium and phosphorus concentrations suggests the presence of dystrophic calcification.
Tc-99m DPD bone scintigraphy is used primarily for skeletal imaging to detect abnormalities of the bone. However, bone scintigraphy also delineates a wide spectrum of nonosseous disorders. Neoplastic, hormonal, inflammatory, ischemic, traumatic, excretory, and artifactual entities demonstrate abnormal soft-tissue uptake of Tc-99m DPD. Mechanisms leading to increased extraosseous Tc-99m DPD uptake include extracellular fluid expansion, enhanced regional vascularity and permeability, and elevated tissue calcium concentration.
Bone scintigraphy can show gastric uptake in various conditions, including artifact due to Tc-99m-pertechnetate in radiopharmaceutical preparation, hypercalcemia due to metastatic calcinosis, dialysis patients and gastric carcinoma. In our case, because the thyroid was not visible, visualization of the stomach on the bone scintigraphy cannot be attributed to a faulty preparation containing free Tc-99m-pertechnetate (9).
There are a few cases showing gastric uptake of tracer in advanced gastric cancer patients (10). However, the fact that the extent of tracer uptake is more diffuse and broader than that of gross calcifications seen on CT images makes our case unique. This may suggest the presence of micro-calcifications in the gastric wall which is not seen on CT images but is detected in bone scintigraphy.
In summary, we describe a rare case showing gastric uptake of Tc-99m DPD in an advanced gastric cancer patient. The extent of tracer uptake in the gastric walls was more diffuse and broader than that of gross calcifications seen on CT images. Calcified gastric cancer is rare, but it was possible to detect even micro-calcifications with Tc-99m DPD bone scintigraphy. As exemplified by this case, recognition of extra-skeletal Tc-99m DPD uptake may enhance the diagnostic value of bone scintigraphy.
Figures and Tables
Fig. 1
Non-contrast enhanced CT of the upper abdomen shows diffuse thickening of gastric antral wall with punctate calcifications (arrows).
References
1. Rotondo A, Grassi R, Smaltino F, Greco A, Leung AW, Allison DJ. Calcified gastric cancer: report of a case and review of the literature. Br J Radiol. 1986. 59:405–407.
2. Balestreri L, Canzonieri V, Morassut S. Calcified gastric cancer-CT findings before and after chemotherapy. Case report and discussion of the pathogenesis of this type of calcification. Clin Imaging. 1997. 21:122–125.

3. Peller PJ, Ho VB, Kransdorf MJ. Extraosseous Tc-99m MDP uptake: a pathophysiologic approach. Radiographics. 1993. 13:715–734.

4. Gruber GB. Knochebildung in einem magen karzinom. Z Beitr Path Anat. 1913. 55:368–370.
5. Kunieda K, Okuhira M, Nakano T, Nakatani S, Tateiwa J, Hiramatsu A, Mizuno T, Shiozaki Y, Sameshima Y. Diffuse calcification in gastric cancer. J Int Med Res. 1990. 18:506–514.

6. De Carvalho JC, Francischetti EA, De Barros Filho GA, Cerda JJ. Calcified mucinous adenocarcinoma of the stomach. Am J Gastroenterol. 1978. 69:481–484.
7. Adachi Y, Mori M, Kido A, Shimono R, Maehara Y, Sugimachi K. A clinicopathologic study of mucinous gastric carcinoma. Cancer. 1992. 69:866–871.

8. Ashley DJB. Evan's Histological Appearances of Tumors. 1990. Edinburgh: Churchill Livingstone;307–308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download