Abstract
Transplant arteriosclerosis is the main limitation for long-term survival of solid organ transplant recipients. Animal models would provide invaluable tools to investigate the cellular and molecular mechanisms underlying the pathogenesis of transplant arteriosclerosis, as well as for studies with novel drugs and other reagents for the prevention of the disease. We have therefore developed a modified technique for aortic transplantation in mice. The central suture ligation of the recipient abdominal aorta allowed a simpler end-to-side anastomosis of a segment of the donor thoracic aorta into the infrarenal portion of the recipient abdominal aorta. Using this technique, the overall survival rate was 94%. We also observed typical aspects of chronic rejection of the aortic allografts not observed with isografts. Our new technique is relatively easy to perform and has a low incidence of thrombosis, thus being useful for studying various aspects of transplant arteriosclerosis.
Mouse aortic transplantation is a model of chronic rejection and transplant arteriosclerosis. Although rat models are popular, mouse models have the advantages of the availability of diverse inbred strains and mutants, as well as the availability of immunological tools. A high level of microsurgical skill is required to perform aortic transplantation in mice, as the diameter of the vascular lumen for anastomosis is less than 0.4 mm. In contrast, the diameter is approximately 8.00 mm in rats, which may be the primary reason that mouse models are not as popular as rat models. In mice the occlusion time of the lower half of the recipient's circulation is minimized within 60 min, which may reduce postoperative mortality from circulatory complications and ischemic paraplegia.
To date, three types of mouse model have been established. In the first, transplant arteriosclerosis was induced by suturing end-to-side of vessel segments to the carotid arteries (1). The second model involved murine heterotopic aorta transplantation (2), but the technical problems, associated with the anastomosis of small vessels in this model, led to the development of improved techniques for heterotopic or orthotopic aortic transplantation (3, 4). In the present study, we developed a modified surgical technique with several advantages compared with the original surgical method (3).
Adult male mice, 8-10 weeks of age, were purchased from Korea Charles River (Seoul, Korea). BALB/c (H-2d) mice were used as donors and C57BL/6 as recipients of aortic allografts.
All operative procedures were performed under intraperitoneal anesthesia using an operating microscope (Olympus, Tokyo, Japan). A stock anesthetic solution was made by mixing fentanyl, midazolam, and droperidol (Samwon Pharmaceuticals, Seoul, Korea) with normal saline.
A transverse incision of the abdomen was made with scissors, and the intestine was mobilized and placed in the left side to expose the vena cava. About 1 mL of blood was aspirated and 1 mL of heparinized saline (100 U/mL) was injected into the vena cava to flush the aorta. Both sides of the posterior ribs were split, and the diaphragm was divided circumferentially as close to the aorta as possible to expose the thoracic aorta. The right lung and heart were placed in the left chest. The intercostal arteries were dissected out as close to the vertebrae as possible to obtain the long branches. The aorta was then severed proximally at the left subclavian artery and distally at the diaphragmatic hiatus. The severed thoracic aorta was flushed with and placed in heparinized saline at 4℃.
A midline incision was made through the abdomen from the xiphoid to the pelvis, and the abdominal wall was retracted. The bowels were wrapped in gauzes soaked with saline and were displaced to the left side. After a midline abdominal incision, the segments of the aorta and vena cava between the left renal vessels (proximally) and their bifurcations (distally) were set free by ligating the spinal vessels underneath the aorta and vena cava with 7-0 black silk. To sequester the aorta and vena cava, the proximal ends were held by a micro-hemostat clamp (Fine Science Tools, Vancouver, BC, Canada) and the distal ends were ligated with 7-0 single silk to allow it to be easily untied after the completion of anastomosis. The middle of the aorta was sutured with 10-0 nylon (Fine Science Tools) to divide the proximal and distal anastomotic areas without severing (Fig. 1A). This central suture ligation allowed us to easily make sequential aortotomies at the proximal and distal parts (Fig. 1B). If this central suture ligation had not been made, blood would be shed after the first aortotomy, making it difficult to make the second. The proximal and distal aortotomies were performed sequentially using the needle tip of a 31-gauge syringe (BD Medical Systems, Singapore) and microscissors (Fine Science Tools). The magnitude of these incisions was determined by the diameter of the harvested donor aorta. An end-to-side anastomosis was established between the donor and recipient aortas, using a running 10-0 nylon suture (Fig. 1C). At the end of this procedure, the distal suture was gently untied, and the proximal clamp was released with the clamp holder. If there was a leakage in the intercostal branches or the anastomotic sites, the proximal end was reclamped and the compression of the bleeding sites was performed with gel-form and cotton swabs for hemostasis. The compression and clamp releasing was repeated until complete hemostasis. After confirming the patency of the grafted aorta (Fig. 1D), the abdominal contents were returned to the abdominal cavity, and the wound was closed with a running 5-0 vicryl suture.
The segment of the grafted aorta between the proximal and distal sutures was harvested 7-70 days after transplantation. Each graft was subsequently divided into two segments of commensurate length. One segment was fixed in 10% buffered formalin and embedded in paraffin after dehydration. Routinely 5 µm cross sections were stained with hematoxylin and eosin (H&E) or silver, using standard procedures. The other segment was used for RNA extraction.
RNA preparation, cDNA synthesis, PCR, and product analysis were carried out as described previously (5).
Fifty aortic grafts (5 syngeneic and 45 allogeneic) were performed using an end-to-side anastomosis modified from the previously developed technique (3). After overcoming the initial technical difficulties, the overall graft survival rate was 94% (47 of 50). The 3 technical failures were caused by thrombosis, the major cause of previous graft failures (2, 3). All of the aortic grafts found patent within 12 hr postoperatively were patent at subsequent follow-up.
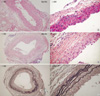
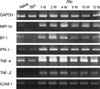
The syngeneic aortic grafts did not show any morphological aberrations associated with graft rejection when examined at various times after transplantation (Fig. 2A). In the allografts, however, we observed a diffuse infiltration of mononuclear cells but no evident neointimal layer 2 weeks after transplantation. By week 4, the allografts exhibited a prominent remodeling pattern with a thick neointimal layer and broken elastin fibers in the media, features observed in chronic rejection (Fig. 2B, C). RT-PCR analysis consistently demonstrated the upregulation of molecules involved in chemotaxis, costimulation, inflammation, tissue remodeling, and cell adhesion (Fig. 3). Overall, the observed arteriosclerotic changes reflected the process of chronic rejection.
Murine aortic transplantation is a useful model for studying chronic allograft and xenograft rejection because the pathological processes observed in the rejected grafts reflect those observed during chronic rejection in humans. Among the techniques used for aortic transplantation are an end-to-end suturing technique for heterotopic (2) and orthotopic (6) aortic transplantation. Lower limb paralysis, however, limits the widespread use of this approach. The use of an orthotopic sleeve technique results in significantly less thrombosis, probably because of the short operation time (4), but the overall survival rate is relatively low. An end-to-side suturing technique has resolved problems associated with size discrepancies between the donor thoracic and recipient abdominal aortas and has markedly increased survival rates (3).
Our modified technique for heterotopic aortic transplantation has several advantages compared with the original technique (3). The suture ligation at the mid-portion of the recipient aorta makes aortotomy easy at the proximal and distal parts of the recipient aorta. Central suture ligation also allows us to omit ligating the segment of the donor abdominal aorta between the anastomotic sites at both ends and severing it at the middle to convert an end-to-side to a quasi end-to-end anastomosis. Postoperative mortality caused by circulation complications and ischemic paraplegia was not a significant problem, due to the shortening of the operation time. We routinely used longer segments of the donor thoracic aorta (over 1 cm) than those used in other techniques, making it possible to obtain a good specimen.
In conclusion, we have developed a simple and reliable technique for aortic transplantation in mice. Since aortic grafts implanted across allogeneic barriers showed the characteristics of chronic rejection observed in humans, this model would be good for studying the basic and clinical problems related to chronic rejection.
Figures and Tables
Fig. 1
Operative steps. (A) Suture ligation of the middle portion of the aorta. (B) Easy aortotomies at the proximal and distal parts of the recipient aorta due to central suture ligation. (C) Completion of the anastomosis. (D) Patency of the grafted aorta.

References
1. Shi C, Russell ME, Bianchi C, Newell JB, Haber E. Murine model of accelerated transplant arteriosclerosis. Circ Res. 1994. 75:199–207.

2. Koulack J, McAlister VC, Giacomantonio CA, Bitter-Suermann H, MacDonald AS, Lee TD. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 1995. 16:110–113.

3. Sun H, Valdivia LA, Subbotin V, Aitouche A, Fung JJ, Starzl TE, Rao AS. Improved surgical technique for the establishment of a murine model of aortic transplantation. Microsurgery. 1998. 18:368–371.

4. Dambrin C, Calise D, Pieraggi MT, Thiers JC, Thomsen M. Orthotopic aortic transplantation in mice: a new model of allograft arteriosclerosis. J Heart Lung Transplant. 1999. 18:946–951.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download