Abstract
Orientia tsutsugamushi causes scrub typhus, which is endemic in many countries in the Asia-Pacific region including Korea. Recent emergence of doxycycline-resistant strains from Thailand has underlined the importance of the susceptibility tests of O. tsutsugamushi to antibiotics. To improve the flow cytometric technique for the susceptibility test, we applied a monoclonal antibody (MAb) in the quantification of O. tsutsugamushi. With using MAb FS15, we determined the doxycycline susceptibility of two strains, Boryong and AFSC-4 strain which is reported to be doxycycline-sensitive and resistant, respectively. The growth of both strains was inhibited to below 10% of the control in the presence of 0.1 µg/mL or higher concentrations of doxycycline. We suggest that our approach is more quantitative and reproducible than the conventional microscopic methods.
Orientia tsutsugamushi causes scrub typhus, which is endemic in many countries in the Asia-Pacific region, including Korea (1, 2). This disease is an acute febrile illness characterized by fever, rash, and eschar. The mechanism of its pathogenesis has not been studied extensively, but is believed to be similar to that of other Rickettsia, which includes endothelial damage and subsequent vasculitis (3).
Doxycycline has been used as the drug of choice for scrub typhus. However, recent reports of doxycycline-resistant strains in Thailand have stimulated the research on alternative antibiotics for the treatment of this disease (4). Because the incidence of scrub typhus has recently increased markedly, there is growing concern about the possible occurrence of a resistant strain in Korea. Therefore, it is necessary to test the susceptibility of Korean isolates of O. tsutsugamushi to doxycycline and other antibiotics (5).
The testing of O. tsutsugamushi antibiotic susceptibility has not been standardized because it requires viable host cells for bacterial growth. In a previous study, we reported the characteristics of a monoclonal antibody (MAb), M686-13, that reacts exclusively with intracellular O. tsutsugamushi, and its application in an immunofluorescence assay (IFA) to determine antibiotic susceptibility (6, 7). However, that method requires the manual count of bacterial particles or computer-assisted scanning. Therefore, we were interested in finding an objective and convenient method with which to accurately quantify O. tsutsugamushi.
Flow cytometry has been used previously for antibiotic-susceptibility testing of intracellular pathogens (8-13). A pioneering work by Kelly et al. described the use of flow cytometry to measure the growth of O. tsutsugamushi in cells treated with antibiotics (14). Although their method is relatively rapid and objective, it is not sufficiently sensitive to distinguish quantitatively between low levels of infection. Moreover, it may be less sensitive for the detection of diverse local strains that are antigenically heterogeneous (14). We speculated that low sensitivity resulted from the use of polyclonal animal serum to stain the bacteria. Therefore, we decided to improve the efficiency of flow cytometric detection by using a specific MAb.
In this study, we demonstrate an improved flow-cytometric approach to the sensitive and quantitative measurement of the growth of O. tsutsugamushi. We used our method to determine the doxycycline susceptibility of strains from Korea and Thailand.
Cells of the human umbilical-vein-derived endothelial cell line ECV304 were maintained in M199 medium (Gibco BRL, Gaithersburg, MD, U.S.A.) supplemented with 10% (v/v) heated-inactivated fetal bovine serum (Gibco BRL) in a humidified atmosphere containing 5% CO2 at 37℃. The Boryong serotype of O. tsutsugamushi (15) was cultivated in ECV304 cells, as described previously (16). The Thailand strains (AFSC-4, AFSC-7, TA686, TA678, TA716, TA763, and TH1817) were kindly provided by Dr. Daniel Strickman, Naval Medical Research Institute, U.S.A.. The Karp (ATCC VR-150) and Gilliam (ATCC-VR-312) strains were obtained from American Type Culture Collection; Kato strain was donated from Dr. Hiroshi Tanaka, the Institute of Medical Science, Tokyo University, Japan. Kuroki and Kawasaki strains were donated from Dr. Akira Tamura, Department of Microbiology, Niigata College of Pharmacy, Japan. All bacterial strains were cultivated in ECV304 cells. When infected ECV304 cells showed maximum cytopathic effects, the cells were disrupted with glass bead (diameter 1.0 mm) and centrifuged at 300×g for 5 min. The resulting supernatants were immediately used to infect further ECV304 cells.
ECV304 cells grown in 6-well plates were incubated with O. tsutsugamushi for 3 hr, allowing time for O. tsutsugamushi to attach to and enter the host cells. At the end of the initial incubation period, the inoculums were replaced with fresh medium containing two-fold dilutions of doxycycline (Sigma, St. Louis, MO, U.S.A.) from 0.2 µg/mL to 0.00625 µg/mL, and the cells were then re-incubated in 5% CO2 at 37℃ for three days. Doxycycline was prepared in aliquots of 0.5 mL at an active concentration of 5,000 µg/mL in sterile distilled water. These aliquots were stored frozen at -20℃ until required.
MAb FS15 and FS10 react against a linear epitope on 56-kDa major outer membrane protein of O. tsutsugamushi (17). Other MAbs (Rb167, Rb134, M686-8, and Shim107) were obtained from cell fusion experiments of spleen cells of mice immunized with live O. tsutsugamushi, as described previously (6, 18). For its application to flow cytometry, MAb FS15 was purified on an AffinityPak Protein A Column (Pierce Biotechnology Rockford, IL, U.S.A.) and conjugated with fluorescein isothiocyanate (FITC) with EZ-label Fluorescein Protein Labeling Kit (Pierce Biotechnology), according to the manufacturer's instruction.
To select a suitable MAb for the application in flow cytometry, we tested the immunoreactivities of MAbs to various strains of O. tsutsugamushi using IFA. Among the tested MAbs, we selected the MAb that had broad reactivity to many strains and stained the bacteria brightly.
The reactivities of MAbs to various strains of O. tsutsugamushi were examined using IFA. ECV304 cells infected with each strain were fixed with acetone-methanol (1:1) or acetone and treated with dilutions of MAbs in phosphate-buffered saline (PBS) for 1 hr at 37℃. After the cells had been washed briefly with PBS, they were treated with FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, U.S.A.) for 1 hr in a moist chamber, and then washed three times with PBS. To clearly define the O. tsutsugamushi, the host cells were counter-stained with 0.003% Evans blue in PBS. After the slides had been washed, they were placed on mounting medium (Vector Laboratories, Burlingame, CA, U.S.A.) and examined under a fluorescence microscope (Nikon, Japan) with a confocal laser scanning system (Bio-Rad, Hercules, CA, U.S.A.).
ECV304 cells infected with O. tsutsugamushi were grown for seven days and were fixed with 0.5% glutaraldehyde, 4% paraformaldehyde and 3.5% sucrose and postfixed with 1% osmium tetroxide (OsO4). After dehydration in an ethanol series, the pellets were embedded in Epon 812. After the collection of ultrathin sections on Formvar/carbon-coated nickel grids, the grids were floated on drops of 3% sodium metaperiodate for 30 min. The immunogold labeling procedure (19) was done using MAb FS15 and 15 nm gold-conjugated goat anti-mouse IgG. After immunogold labeling, the grids were stained with uranyl acetate and lead citrate, and was viewed with a Hitachi H7100 electron microscope.
To quantify O. tsutsugamushi in ECV304 cells, infected cells were trypsinized, washed with PBS, and centrifuged at 300×g for 5 min. The cells were fixed with 70% ethanol for 1 hr on ice and stored at -20℃ until analyzed. Before their application to flow cytometry, fixed cells were washed with PBS and the resulting cell pellets were resuspended in 500 µL of FITC-conjugated MAb FS15 diluted with PBS, and analyzed with a flow cytometer (Becton Dickinson, Mansfield, MA, U.S.A.) equipped with a 15 mW argon laser. Data were collected on 10,000 cells and analysis was performed using LYSIS II software (Becton Dickinson). To calculate an estimate of the total mass of bacteria in the cells, the percentage of positive cells was multiplied by the mean fluorescence intensity of these cells to obtain the growth index (GI). The MIC was defined as the concentration of antibiotics at which GI was reduced by 90% relative to that of control cells that were infected and incubated in the absence of doxycycline.
The tested MAbs could stain O. tsutsugamushi brightly in IFA (Fig. 1A). Although the MAbs showed similar morphologies of bacteria, their staining patterns differed slightly according to the target antigens. In addition, the bacterial particles stained by MAbs (FS10, FS15, and Rb167) directed against the 56-kDa scrub typhus antigen (Sta56) were brighter and bigger than those stained by MAbs (Rb134, M686-8, and Shim107) directed against other antigens. Therefore, we reasoned that MAbs against Sta56 are suitable as the primary antibodies for the use in flow cytometry. Among them, we selected FS15 because the target epitope of this MAb has been well characterized in a previous study (17). Furthermore, the antigenic epitope is located at the surface of the cell envelope as shown by immunogold electron microscopy (Fig. 2A). As Sta56 has strain-specific and group-specific epitopes, we tested the reactivity of FS15 to other strains of O. tsutsugamushi. As shown in Fig. 2B. MAb FS15 stained most of the O. tsutsugamushi strains (Boryong, Karp, Kato, AFSC-4, AFSC-7, TA686, TA763, TH1817, Kawasaki and Kuroki) originating from several Asian nations in IFA, except Gilliam, TA678, and TA716 strains.
To test the applicability of FITC-conjugated FS15 to the quantification of the total mass of O. tsutsugamushi by flow cytometry, we first used this MAb to determine the growth of O. tsutsugamushi. We infected the ECV304 cells with high (84.6% cells are infected 3-day postinfection) and low numbers (12.2% cells are infected 3-day postinfection) of O. tsutsugamushi and measured bacterial growth for seven days using flow cytometry. Under both conditions, flow cytometry demonstrated the significant growth of O. tsutsugamushi, which appeared at as early as one day after infection and progressed further until seven days after infection. This is evident in the right-hand shift of the entire peak of cells infected with high numbers of bacteria (Fig. 3A) and the appearance of a new peak in cells infected with low numbers of bacteria (Fig. 3B). Because three days incubation was sufficient to demonstrate bacterial growth by flow cytometric parameters, we selected this time point for subsequent work.
To test the applicability of flow cytometry to the determination of the doxycycline susceptibility of the Boryong strain, which is the most prevalent strain in Korea (15), infected ECV304 cells were treated with various concentrations of doxycycline for three days, and then bacterial growth was measured by flow cytometry. As shown in Fig. 4A, the fluorescence peak shifted to left as the concentration of doxycycline increased, as indicated by the percentage of positive cells in each histogram. The percentage of positive cells infected with Boryong after treatment with 0.1 µg/mL doxycycline was 1.7% (Fig. 4A). In the presence of 0.1 or 0.2 µg/mL doxycycline, no bacterial growth was observed and the percentage of positive cells decreased to that of the control (uninfected) cells. Thus, the MIC was determined to be 0.1 µg/mL.
Next, we used our method to determine the doxycycline susceptibility of O. tsutsugamushi strain AFSC-4, which is reported to be resistant to doxycycline (5). The percentage of positive cells infected with AFSC-4 after treatment with 0.1 µg/mL doxycycline was 3.1% and the MIC was 0.1 µg/mL (Fig. 4B). When we compared the doxycycline susceptibility of the Boryong and AFSC-4 strains, both strains showed similar patterns of inhibition by doxycycline. The percentages of GI of Boryong and AFSC-4 strains after the treatment with 0.1 µg/mL doxycycline were 1.29% and 4.11% respectively, where 100% represented the percentage of infected cells without doxycycline treatment (Fig. 4C).
In this study, we have demonstrated that flow cytometry using specific MAb can be used to reliably quantify the total mass of O. tsutsugamushi. Traditional methods of quantifying other Rickettsia, such as the determination of the reduction in plaque formation or the dye-uptake assay (20), are seldom used for Orientia because it requires a long incubation time to form plaques (21). The most frequently used method involves counting Giemsa-stained bacteria after 3-4 days of antibiotic treatment (5). Whereas this method requires few special reagents, it is time-consuming and complicated owing to difficulties in quantifying the bacterial particles microscopically because of their tendency to form aggregates in cells (22, 23). With our method using flow cytometry, a right-hand shift of the entire peak or the appearance of a new peak was observed as O. tsutsugamushi divided. These results were in contrast to a previous trial using flow cytometry, in which only a slight right-hand shift occurred in the fluorescence peak of infected cells and seemed to be essentially qualitative rather than quantitative (14).
The MAb is the most important parameter in the sensitive detection of O. tsutsugamushi by flow cytometry. For the detection of bacteria in cells by flow cytometry, a primary antibody must have several properties: 1) it must be species-specific to detect various strains of O. tsutsugamushi, which is particularly important when we consider the antigenic heterogeneity of this bacterium; 2) target antigen should be expressed in large amounts, so that the antibody stains bacteria brightly; and 3) it must be available in large amounts so that the same reagent can be used in future applications.
We used a mouse MAb that is reactive against Sta56 of O. tsutsugamushi. This protein is abundantly expressed at the surface of the bacterium and is believed to be important in its pathogenesis (1). It has strain-specific and species-specific epitopes and is a major target of strain-specific immunity to O. tsutsugamushi. MAb FS15 was well characterized in our previous study as being reactive against amino acid residues 187-214 of Sta56. MAb FS15 was also reactive strongly on IFA against various strains of O. tsutsugamushi, including the most prevalent strain in Korea (15). Therefore, it can be used to test the antibiotic susceptibility of most strains. The morphologies of the bacteria stained with various MAbs on IFA differed according to the target antigen of each MAb. Interestingly, MAbs directed against Sta56 showed bigger and brighter bacterial particles than those detected by MAbs against other antigens of O. tsutsugamushi (Fig. 1). This is one of the desirable properties of an antibody to be used with flow cytometry. Our explanation for this finding is that Sta56 is an abundantly expressed protein at the cell surface of this bacterium. Of the MAbs directed against Sta56, we chose FS15 because it shows minimal nonspecific staining of host cells (data not shown).
The MIC of doxycycline was 0.1 µg/mL for both the Boryong and AFSC-4 strains when we measured bacterial growth after treatment with doxycycline for three days. This result is consistent with that of our previous study, in which we determined the antibiotic susceptibility of both strains using IFA (7). However, strain AFSC-4 is reported to be doxycycline-resistant and its MIC was reported to be 0.25 µg/mL (5). Although our approach produced an unexpected result, we would like to point that our method using flow cytometer is more objective than the microscopic count of Giemsastained bacterial particles used by authors of report for AFSC-4 strain (5). Although clinical experiences support the occurrence of infections by strains that are poorly responsive to doxycycline in northern Thailand, there are few reports about the biology of resistant strains and their mechanism for resistance. Therefore, further studies are required to clarify the mechanisms of doxycycline resistance of O. tsutsugamushi and the possible emergence of doxycycline-resistant in Korea.
In summary, we have improved the flow cytometric technique for the quantification of O. tsutsugamushi growth using specific MAb directed against Sta56. Our approach is sensitive in measuring the growing bacteria inside the cells. With this method, we measured the doxycycline susceptibility of the Boryong and AFSC-4 strains.
Figures and Tables
Fig. 1
IFA staining of MAbs Boryong strains of O. tsutsugamushi. MAbs directed against a Sta56 show brighter and bigger bacterial particles than those seen by MAbs against other antigens (×80).
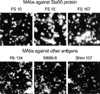
Fig. 2
Reactivity of MAb FS15 selected for the application in flow cytometry. (A) Immunogold staining of O. tsutsugamushi with MAb FS15 shows that epitope of FS15 was located at the surface of the cell envelope (×25,000). (B) MAb FS15 reacts positively against 10 strains out of 13 strains from various nations (×20).
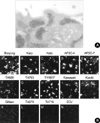
Fig. 3
Flow cytometric histograms of ECV304 cells infected with a high dose (A) or low dose (B) of Boryong strain at the indicated times. Control represented the result from uninfected cells. The percentages of positive cells for O. tsutsugamushi are marked in the histogram.

Fig. 4
Comparison of susceptibility of the Boryong and AFSC-4 strains to doxycycline. Flow-cytometric histograms of ECV304 cells infected with the Boryong (A) or AFSC-4 strain (B) in the presence of increasing concentrations of doxycycline (0-0.2 µg/mL) were measured after three day incubation. Control represents the result from the uninfected cells. The histograms are representative of three independent experiments. The GI of each strain was calculated and plotted as a percentage of fluorescence intensity (C), where 100% represents the percentage of infected cells without doxycycline treatment. Bars represent means±SD (n=3).

References
1. Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001. 3:11–21.
3. Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001. 14:752–759.

4. Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, Strickman D. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996. 348:86–89.

5. Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995. 39:2406–2410.

6. Kim MK, Odgerel Z, Chung MH, Lim BU, Kang JS. Characterization of monoclonal antibody reacting exclusively against intracellular Orientia tsutsugamushi. Microbiol Immunol. 2002. 46:733–740.
7. Kim MK, Odgerel Z, Kim MJ, Chung MH, Lim BU, Kang JS. Application of monoclonal antibody, specific for intracellular Orientia tsutsugamushi, to immunofluorescent antibody test for determining antibiotic susceptibility. Microbiol Immunol. 2004. 48:655–660.
8. Alvarez-Barrientos A, Arroyo J, Canton R, Nombela C, Sanchez-Perez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000. 13:167–195.

9. Dessus-Babus S, Belloc F, Bebear CM, Poutiers F, Lacombe F, Bebear C, de Barbeyrac B. Antibiotic susceptibility testing for Chlamydia trachomatis using flow cytometry. Cytometry. 1998. 31:37–44.
10. Levitt D, Zable B, Bard J. Binding, ingestion, and growth of Chlamydia trachomatis (L2 serovar) analyzed by flow cytometry. Cytometry. 1986. 7:378–383.
11. Matyszak MK, Young JL, Gaston JS. Uptake and processing of Chlamydia trachomatis by human dendritic cells. Eur J Immunol. 2002. 32:742–751.

12. Waldman FM, Hadley WK, Fulwyler MJ, Schachter J. Flow cytometric analysis of Chlamydia trachomatis interaction with L cells. Cytometry. 1987. 8:55–59.
13. Messick JB, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993. 61:3803–3810.

14. Kelly DJ, Salata KF, Strickman D, Hershey JN. Rickettsia tsutsugamushi infection in cell culture: antibiotic susceptibility determined by flow cytometry. Am J Trop Med Hyg. 1995. 53:602–606.

15. Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990. 28:685–688.

16. Kim MK, Kee SH, Cho KA, Chung MH, Lim BU, Chang WH, Kang JS. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 1999. 43:751–757.
17. Seong SY, Kim MK, Lee SM, Odgerel Z, Choi MS, Han TH, Kim IS, Kang JS, Lim BU. Neutralization epitopes on the antigenic domain II of the Orientia tsutsugamushi 56-kDa protein revealed by monoclonal antibodies. Vaccine. 2000. 19:2–9.

18. Kang JS, Chang WH. Antigenic relationship among the eight prototype and new serotype strains of Orientia tsutsugamushi revealed by monoclonal antibodies. Microbiol Immunol. 1999. 43:229–234.
19. Spector DL, Fu XD, Maniatis T. Associations between distinct premRNA splicing components and the cell nucleus. EMBO J. 1991. 10:3467–3481.

20. Raoult D, Roussellier P, Vestris G, Tamalet J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: plaque assay and microplaque colorimetric assay. J Infect Dis. 1987. 155:1059–1062.

21. Hanson B. Improved plaque assay for Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1987. 36:631–638.

23. Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, Choi MS. Microtubule and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun. 2001. 69:494–500.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download