Abstract
Intracranial cavernous angiomas are benign vascular malformations and can be divided into intra-axial and extra-axial lesions. Extra-axial cavernous angiomas are relatively rare and usually arise in relation to the dura mater and mimick meningiomas. We report a case of cavernous angioma that occured in the falx cerebri of a 22-yr-old female patient with the special focus on neuroradiologic findings. This is the fourth case of cavernous angioma in the falx cerebri reported in the literature to our knowledge.
The cavernous angioma, also known as the cavernous malformation or cavernoma, is one of the vascular malformations characterized by the presence of sinusoid-like capillary vessels containing blood in very sluggish circulation (1). Intracranial cavernous angiomas are mostly parenchymal lesions and extra-axial cavernous angiomas are relatively rare, accounting for only 0.4 to 2% of all intracranial vascular malformations (2). Extra-axial cavernous angiomas usually arise in relation to the dura mater intracranially or at the spinal level and often mimick meningiomas. Most of these lesions have been found in the middle fossa at the level of cavernous sinus and they are rarely found in other dural locations outside the middle fossa. Other dural locations include the tentorium, the convexity, the anterior cranial fossa, Meckel's cave, the cerebellopontine angle, the sellar turcica, internal auditory canal, and cranial nerves (2-4). Only 3 cases of cavernous angiomas originated from the falx cerebri have been reported in the literature (3, 5, 6). We report a case of cavernous angioma occured in the falx cerebri with the neuroradiologic features, which is the fourth case to our knowledge.
A 22-yr-old female patient presented with an episode of generalized tonic-clonic type seizure. She had no focal neurologic deficit.
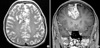
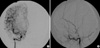
Computerized tomography (CT) scan showed a 5×6-cm, well-defined, and lobulating iso-attenuation mass in the right frontal region with dense and scattered calcifications. The mass showed mild enhancement following contrast media administration (Fig. 1). Magnetic resonance image (MRI) showed an extra-axial lobulating lesion abutting on the right to the falx with slightly hypointense signal intensity on T1-weighted image (T1W) and mixed hyper- and hypointense signal intensity on T2-weighted image (T2W). Following administration of gadolinium contrast agent, mass showed heterogeneous enhancement with dural tail sign. Multiple dot-like and linear signal voids were also seen in the mass (Fig. 2). Angiography showed the displacement of anterior portion of the both anterior cerebral arteries to the left side. No definite abnormal capillary blush or malformed vessels was seen on the right external cerebral artery or internal cerebral artery angiography (Fig. 3). There was no abnormal hot uptake lesion in the brain Thallium201 SPECT scan.
When the dura was opened, thickening of the dura was noticed. The surrounding brain parenchyma was gliotic and stained yellowish by hemosiderin. The mass was originated from the falx, having a various consistency from soft to hard with areas of thrombosis and calcifications. Even though the lesion was highly vascular, there was no difficulty in bleeding control and it was totally excised en bloc using microsurgical techniques.
A histologic examination showed numerous large, dilated, blood-filled vessels lined by a flattened endothelium with a single cell layer, and marked calcification within the mass as well. Vascular channels were separated by fibroconnective tissue stroma. The vascular spaces within the lesion were predominantly occupied by numerous capillaries (Fig. 4).
Histopathologically, intracerebral and extra-axial cavernous angiomas seem to be identical lesions. Cavernous angiomas consist of abnormally dilated, thin-walled vascular channels with walls composed of collagen lined by a layer of endothelium. Surrounding brain parenchyma is often gliotic and hemosiderin-stained and the lesions may contain feeding arteries and draining veins of slow flow. It can vary from a small petechial lesion of a few millimeters to a larger, well-circumscribed, ''mulberry-like'' hemorrhagic mass of many centimeters in size (1, 2, 7, 8).
Intracranial cavernous angiomas are generally found in middle-aged women (3), though its cause is uncertain. Previously reported 3 cases of cavernous angiomas of falx cerebri were found in 47, 62, and 63-yr old women (3, 5, 6), respectively.
Though there is no specific neuroradiologic finding for extra-axial cavernous angiomas, the MRI pattern was known to reflect the histological appearance (7). The most common form of MRI findings such as speckled mixed intensity may reflect vascular lumens, their thromboses, and calcifications in the lesion. Intra-axial cavernous angiomas usually have a multifocal hyperintense center surrounded by a hypointense rim which results from the blood products in different stages of evolution (1, 9-11). However, the characteristic peripheral low-signal rim is usually absent in extra-axial cavernous angiomas, and this was true in our case. Extra-axial cavernous angiomas also have been reported as a soft mass often with a pseudocapsule that is formed by the dura mater, mimicking meningiomas.
Guermazi et al. (12) demonstrated that dural tail sign could be rarely seen in cavernous angioma from the dura in the cerebral convexity, and differential diagnosis from meningioma is even more difficult to eliminate when the dural cavernous angioma shows a dural tail. In our case, dural tail sign and calcifications were very prominent (3, 13).
In intracranial cavernous angioma, angiography usually reveals the flecked lesion stain at middle arterial to late venous phases due to slow blood flow, delayed stain, or an avascular mass. The sunburst of vessels radiating outwards from the central vascular pedicle, a typical finding of meningiomas, was reported that it was not found in cavernous angioma (3, 6, 8). Only an avascular mass effect was present in our case.
Little is known about the role of Thallium201 SPECT in the diagnosis of cavernous angioma. Seo et al. (14) reported that Thallium201 SPECT is useful in diagnosis of cavernous sinus hemangioma, presenting low uptake within the tumor in contrast to meningioma or malignant tumors which present high uptake due to increased tumor viability or tumor blood flow. The uptake of Thallium201 within the brain is associated with tumor viability and tumor blood flow, and is much higher within meningiomas than malignant tumors. In this case, no definite abnormal Thallium201 accumulation was observed, as same as the result of Seo's study.
In relation to the surgery of cavernous angioma at the cavernous sinus, bleeding was reported to be a major problem and preoperative differential diagnosis of extra-axial cavernous angioma from meningioma may be most important. However, little blood loss was reported in the surgery of cavernous angiomas of the falx cerebri (6). In our case, blood loss was only a little, and total removal was achieved without difficulty. No adjuvant therapy was required during the follow-up period.
We reported a very rare case of cavernous angioma that occured in the falx cerebri which was successfully removed, with special interest on its neuroradiologic features.
Figures and Tables
Fig. 1
Pre- (A) and post contrast (B) CT scans show a well-defined, lobulating, iso-attenuation mass in the right frontal region with dense and scattered calcifications in the central and peripheral regions, respectively. The mass shows no definite enhancement except for anterior falx enhancement following contrast media administration.
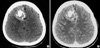
Fig. 2
Axial T2-weighted magnetic resonance (MR) (A) and coronal T1-weighted MR gadolinium-enhanced (B) images show an extra-axial, lobulating lesion abutting on the right side to the falx cerebri with heterogeneous enhancement with dural tail sign, CSF cleft, and cortical buckling. Multiple dot-like and linear signal voids are also seen in the mass.
References
1. Curling OD Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991. 75:702–708.
2. Zabramski JM, Awasthi D. Awad IA, Barrow DL, editors. Extra-axial cavernous malformations. Cavernous malformations. 1993. Park Ridge, IL: American Association of Neurological Surgeons;133–144.
3. Biondi A, Clemenceau S, Dormont D, Deladoeuille M, Ricciardi GK, Mokhtari K, Sichez JP, Marsault C. Intracranial extra-axial cavernous (HEM) angiomas: tumors or vascular malformation? J Neuroradiol. 2002. 29:91–104.
4. Lee SB, Lee JI, Kim JS, Hong SC, Park K. Treatment of brainstem cavernomas. Korean J Cerebrovasc Surg. 2004. 6:58–63.
5. Fracasso L. Considerazioni su un caso di angioma della falce. Riv Patol Nerv Ment. 1947. 68:214–226.
6. Kaga A, Isono M, Mori T, Kusakabe T, Okada H, Hori S. Cavernous angioma of falx cerebri; case report. No Shinkei Geka. 1991. 19:1079–1083.
7. Ebeling JD, Tranmer BI, Davis KA, Kindt GW, De Masters BK. Thrombosed arteriovenous malformation. Magnetic resonance imaging and histopathological correlations. Neurosurgery. 1988. 23:605–610.
8. Robinson JR Jr, Awad IA, Masaryk TJ, Estes ML. Pathological heterogeneity of angiographically occult vascular malformations of the brain. Neurosurgery. 1993. 33:547–555.

9. Harper DG, Buck DR, Early CB. Visual loss from cavernous hemangiomas of the middle cranial fossa. Arch Neurol. 1982. 39:252–254.

10. Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). J Neurosurg. 1987. 67:518–524.

11. Sato M, Nakano M, Sasanuma M, Asari J, Watanabe K, Hanai K. MR image of multiple intracranial cavernous angioma: the usefulness of gradient-echo MR image. No To Shinkei. 2003. 55:172–173.
12. Guermazi A, Lafitte F, Miaux Y, Adem C, Bonneville JF, Chiras J. The dural tail sign-beyond meningioma. Clin Radiol. 2005. 60:171–188.

13. Taniuchi N, Yamazaki M, Katsura K, Igarashi H, Sakamoto S, Katayama Y. T2-weighted MRI in a case with multiple cerebral cavernous malformations. No To Shinkei. 2002. 54:1092–1093.
14. Seo Y, Fukuoka S, Sasaki T, Takanashi M, Hojo A, Nakamura H. Cavernous sinus hemangioma treated with gamma knife radiosurgery: usefulness of SPECT for diagnosis--case report. Neurol Med Chir. 2000. 40:575–578.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download