Abstract
The MHC class II transactivator (CIITA) is the master transcriptional regulator of genes involved in MHC class II restricted antigen presentation. Previously we suggested another role of CIITA in Th1/Th2 balance by demonstrating that forced expression of CIITA in murine T cells repressed Th1 immunity both in vitro and in vivo. However, the results were contradictory to the report that CIITA functioned to suppress the production of Th2 cytokine by CD4+ T cells in CIITA deficient mice. In this study, we investigated the influence of constitutive expression of CIITA in T cells on Th2 immune response in vivo using murine experimental colitis model. In the dextran sodium sulfate-induced acute colitis, a disease involving innate immunity, CIITA transgenic mice and wild type control mice showed similar progression of the disease. However, the development of oxazolone-induced colitis, a colitis mediated by predominantly Th2 immune response, was aggravated in CIITA-transgenic mice. And, CD4+ T cells from the mesenteric lymph node of CIITA-transgenic mice treated with oxazolone exhibited a high level of IL-4 secretion. Together, these data demonstrate that constitutive expression of CIITA in T cells skews immune response to Th2, resulting in aggravation of Th2-mediated colitis in vivo.
Major histocompatibility complex class II transactivator (CIITA), a non-DNA-binding transcription factor, is required for expression of MHC class II and other genes related to antigen (Ag) presentation in both conventional and non-conventional antigen-presenting cells (APCs) (1-3). Upon stimulation with IFN-γ, STAT-1 triggers CIITA expression through interferon-γ activation sequence (GAS) elements present within the CIITA promoter (4). Because CIITA does not bind DNA directly, CIITA must exert its effects through interaction with other proteins bound to the conserved elements within the MHC class II (MHC II) promoter. CIITA binds to the NF-Y and RFX heterodimers and is believed to stabilize the minimal MHC class II enhanceosome, which promotes the recruitment of general transcription factors, RNA polymerase II, and various coactivators including CBP to stimulate expression of the MHC II molecules (5). Although CIITA was initially described as a master regulator of MHC class II transcription, it is now clear that it can both activate and suppress transcription of several genes such as collagen (6), matrix metalloproteinase-9 (7), IL-4 (8), Fas ligand (9) and thyroid specific genes (10, 11). Therefore it seems that the CIITA protein interacts with a variety of transcription factors in the cells and would be involved in diverse functional aspects of immune responses in vivo.
In addition to its control over expression of various genes including MHC class II, CIITA has also been postulated to participate in CD4+ Th cell differentiation. For example, CD4+ T cells from CIITA-/- I-E-transgenic mice produced enhanced levels of IL-4 and other Th2-type cytokines upon activation (12). A subsequent experiment has suggested that the competition between CIITA and NF-AT for binding to CPB/p300 is critical for the suppression of IL-4 transcription by T cells (8). These results supported the notion that CIITA could be a Th1-specific factor that functions as a direct repressor of IL-4 expression. However, our previous results obtained from CIITA-transgenic mice (Dlck-CIITA Tg) were contradictory to the previous ones, in that CD4+ T cells from the Dlck-CIITA Tg mice, in which the CIITA expression is restricted to peripheral T cells, exhibited a low level of IFN-γ secretion as well as impaired Th1 polarization in vitro, while IL-4 secretion was enhanced under the Th2 condition (13). Moreover, development of experimental autoimmune encephalomyelitis (EAE), a prototype of Th1-mediated disease, was repressed in the Dlck-CIITA Tg mice (13). Otten et al. reported that constitutive expression of CIITA in all organs including T cells led to Th2 bias during Th activation and differentiation (14). All together, it could be suggested that overexpression of CIITA in T cells inhibits Th1 differentiation both in vivo and in vitro. To clarify the discrepancies between the results obtained from CIITA deficient mouse and CIITA overexpressing mice regarding the role of CIITA function in Th differentiation, immune reaction induced under Th2 condition should be tested in the CIITA transgenic mice.
Abnormal mucosal T cell responses are related to the development of human inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis. Among the diseases, the development of Crohn's disease has been known to be based on Th1 response, while the ulcerative colitis has been regarded as a representative Th2 response-mediated disease. The Th2-mediated colitis could be induced in mouse models by the intrarectal administration of the haptenating agent oxazolone in an ethanol vehicle (15, 16).
To determine whether CIITA could indeed play a role in promotion of Th2 response in vivo, we induced experimental colitis with oxazolone using Dlck-CIITA-transgenic (CIITA Tg) mice with restricted expression of CIITA gene in T cells. And we show here that administration of oxazolone induced more severe colonic inflammation in the CIITA Tg mice, implying that Th2 dependent immunity is enhanced.
The generation of CIITA transgenic mice on C57BL/6 (B6) background was previously described (13). C57BL/6 mice were purchased from Daehan Biolink (Chungbuk, Korea). All mice were housed in a specific pathogen-free facility in Hallym University. All experimental animals were cared for, maintained, and terminated by CO2 inhalation in accordance with the Hallym University Guideline.
Colitis was induced in CIITA-transgenic mice and littermate wild type B6 mice by addition of 2.5% DSS (dextran sodium sulfate M.W. 36,000-50,000; MP Biomedicals, Irvine, CA, U.S.A.) to drinking water for 12 days. Control mice received drinking water only. Animals were monitored daily for loss of body weight, and survival for 12 days. On day 7 after starting DSS administration, when clinical symptoms such as diarrhea, hematochezia, and weight loss were obvious, mice were sacrificed and colon length was recorded.
Colitis was induced in CIITA-transgenic mice and littermate wild type B6 mice as previously described (16). In brief, mice were sensitized by the epicutaneous application of 200 µL of 3% (w/v) oxazolone (4-ethoxymethylene-2-phenyl-2-oxazoline-5-one, Sigma-Aldrich, St Louis, MO, U.S.A.) solution onto the shaved abdomens. Five days after presensitization, mice were rechallenged intrarectally with 150 µL 1% oxazolone in 50% ethanol or only 50% ethanol (i.e., vehicle) under light anesthesia. Intrarectal injection was administrated with a 3.5 F catheter. Animals were monitored daily for loss of body weight, and survival for 7 days. At 3 days after oxazolone administration, when the difference between the degree of weight loss of wild type and that of CIITA transgenic mice was peak, mice were sacrificed and colon length was recorded.
Mouse CD4+ T cells were harvested from mesenteric lymph nodes of CIITA-transgenic or littermate mice by Ficoll-Paque (Amersham Bioscience, Buckinghamshire, U.K.) gradient centrifugation of lymphocytes and magnetic cell sorting using MACS with anti-CD4 (GK1.5) microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) as recommended by the manufacturer. The purity of collected CD4+ T cells was determined by flow cytometry using anti-CD4 mAb (GK1.5) and ranged from 95 to 97%. The purified CD4+ T cells were stimulated with anti-CD3 mAb (2C11) and 50 µg/mL mitomycin-C treated splenocytes in RPMI medium (Amersham Bioscience) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, U.S.A.) and 50 µM β-mercaptoethanol for 3 days. ELISA used the culture supernatants for cytokine assay.
Quantitative analysis of cytokines in cell culture supernatants was performed by sandwich ELISA using the OptEIA kits (Pharmingen, San Diego, CA, U.S.A.).
Colons were removed, fixed in 10% neutral formalin, embedded in paraffin, sectioned at 4 µm and stained with hematoxylin and eosin (H&E). The severity of lesion was compared among each group by light microscopic examination.
Comparison of the mean cytokine secretion between wild-type and CIITA-transgenic mice were analyzed by the Mann-Whitney's rank sum test. Comparison of the body weight between two groups was done by two-way ANOVA. p values less than 0.05 were considered statistically significant.
Administration of DSS dissolved in water to mice induces acute colitis that occurs while the DSS is administered, and chronic colitis a little time after the administration (17). First, we investigated the effect of CIITA expression in T cells on the course of development of DSS-induced acute colitis. The average weights of the C57BL/6 wild type mice and CIITA-Tg mice on day 0 rights before the DSS administration were 22.9±1.4 g and 23.6±0.9 g, respectively. Then body weight was monitored for following 7 days, and histopathologic features were evaluated with tissue samples on day 7. Clinical symptoms of acute colitis such as a progressive loss of body weight (32.72±0.02% decreases compared with control group treated with water), hematochezia, and diarrhea were noted in the DSS-administered mice; loose stool was detected from day 4 and diarrhea and hematochezia were noted after the 5th day following administration of 2.5% DSS. CIITA-Tg mice showed similar pathway of acute colitis development after DSS administration and the extent of loss of body weight was comparable with that observed in normal mice treated with DSS (Fig. 1). Histopathologic examination of the colon on day 7 after DSS administration showed massive infiltration of mixed inflammatory cells in mucosa and submucosa with epithelial denudation, destruction of crypt architecture, and ulceration (Fig. 2). Pathologic changes in colon induced by DSS administration for 7 days were similar in wild type and CIITA Tg mice (Fig. 2), implying that CIITA expression in T cells did not influence the development of DSS-induced colitis.
Next, we tested whether the over-expression of CIITA in peripheral T cells might influence Th2 immune response in vivo. Administration of oxazolone per rectum in the presence of ethanol induces a mucosal immune response leading to a rapidly developing colitis confined to the distal half of the colon in susceptible (SJL and C57BL/6) mouse strains (15). In this model, the inflammation is characterized by increased IL-4 and IL-5 secretion and the inflammation can be ameliorated by the administration of anti-IL-4 antibodies (15). CIITA-Tg mice and wild type littermates were sensitized by the epicutaneous application of 3% oxazolone solution onto the shaved abdomens, followed by administration of 1% oxazolone per rectum 5 days later. While control animals, which received 50% ethanol in PBS, appeared to have only a transient weight loss without morbidity, oxazolone-challenged B6 mice developed colitis, showing more severe weight loss that peaked 1 day after induction and sometimes culminated in death by day 3 (Fig. 3). This was accompanied by shortening of colonic length; 49±4 mm and 62±4 mm in oxazolone- and vehicle-treated mice, respectively. Microscopic examination of colon extracted from these mice on day 3 after oxazolone challenge per rectum showed histological evidence of severe colitis, which involved only the distal half of the bowel. Oxazolone-treated mice developed dense infiltration of the mucosa with small polynuclear granulocytes and lymphocytes (Fig. 4B). These clinical and pathologic features of oxazolone-induced colitis were significantly aggravated in CIITA-Tg mice. Treatment with oxazolone per rectum led to more severe weight loss and slow recovery in weight loss (Fig. 3A) in the CIITA-Tg mice, although survival rate was not statistically different from that of wild type littermate (p=0.37) (Fig. 3B). Macroscopically, the length of the colon in the oxazolone-treated CIITA Tg group (37±3 mm) was shorter than that in the wild type group treated with oxazolone (49±4 mm) (Fig. 4A). Histological findings showed significantly higher number of inflammatory cells infiltrating from mucosa to serosa of the colons in oxazolone-treated CIITA-Tg mice, compared with wild type controls (Fig. 4B).
As oxazolone-induced colitis is a representative Th2 cell-mediated process, we compared Th1 and Th2 cytokine secretion by CD4+ T cell from CIIT-Tg mice and wild type littermates. When the CD4+ T cells isolated from mesenteric lymph nodes of CIITA-Tg or wild type mice undergoing oxazolone-induced colitis were stimulated with anti-CD3 antibody in ex vivo, CD4+ T cells isolated from CIITA Tg mice produced larger amount of Th2 cytokine, IL-4 but lower level of IFN-γ than those from wild type mice (Fig. 5).
Thus, compared with DSS-induced colitis, the over-expression of CIITA in peripheral T cells clearly showed an adverse effect on the inflammation induced by treatment of oxazolone in colons, which is associated with the Th2 bias in CIITA-expressing CD4+ T cells.
Besides its function as a key regulator for the expression of MHC class II molecule (1, 2), CIITA has been known to play additional roles in Th differentiation and activation-induced cell death (8, 9, 12, 14, 18). Previously, we have shown that the constitutive expression of CIITA in mature T cells suppressed Th1 differentiation both in vitro and in vivo (13). Using the same CIITA-Tg mice, we tested the effect of CIITA in Th2-differentiation in vivo. In this study, we showed that forced expression of CIITA in T cells enhanced Th2 immunity, which was confirmed by the increased susceptibility of CIITA-Tg mice to oxazolone-induced colitis, a Th2-mediated IBD model.
Recently, the contradictory results, whether CIITA inhibits Th2 differentiation or Th1 differentiation, obtained from CIITA knock-out mice or CIITA transgenic mice, respectively were reported. The function of CIITA in Th differentiation was first demonstrated by the observation of increased generation of IL-4 secreting CD4+ T cells in CIITA-/-I-E-transgenic mice (12). Treatment of purified protein derivative (PPD) of Mycobacterisum bovis induced smaller granuloma in the CIITA-/-I-E-transgenic mice, development of which depends on the ability to generate a Th1-dominated immune response (19), than the granuloma in PPD-treated control mice (12). The IFN-γ levels were comparable between the two groups of mice, indicating that PPD-specific T cells had differentiated into Th1 by the sensitization. Therefore, Gourley et al. has suggested that the decrease in the granuloma size in the CIITA-/-I-E-transgenic mice might be due to the elevated IL-4 production, not a decrease in the IFN-γ level. In addition, a transfection study has shown that CIITA might suppress IL-4 transcription in Th1 cells by competing with NF-AT for binding to the general coactivator CBP (8). These data predict that the over-expression of CIITA in T cells should lead to the suppressed Th2 differentiation and an enhanced Th1 differentiation of the CIITA-Tg cells upon activation. However, the data shown by Otten et al. and our group were in sharp contrast with the prediction, in that the two independent CIITA-Tg mice (ectopic expression of CIITA in all types of cells in the body and restricted expression of the molecule in mature T cells, respectively) rendered the transgenic CD4+ T cells to differentiate preferentially into IL-4 secreting Th2-type cells in vitro (13, 14). Our interpretation on the controversy is that the previous studies on the function of CIITA in peripheral CD4+ T cells from CIITA-deficient and -transgenic mice have focused largely on repression of Th1 immunity in vivo, while Th2 bias was checked via in vitro studies. In current study, we evaluated the effect of CIITA expression in mature CD4+ T cells on innate and Th2 immune response in vivo using DSS- or oxazolone-induced colitis model.
The administration of DSS dissolved in water to mice caused hematochezia, body weight loss, shortening of the intestine, mucosal ulcers, and infiltration of neutrophils (17). This acute colitis, which occurred during the administration of DSS, was considered to be induced by innate immunity, but not acquired immunity (20). Based on our results, CIITA expression in T cells did not have any influence on the DSS-induced innate immunity, acute colonic inflammation, resulting in similar progression of the disease both in CIITA Tg and B6 wild type mice.
Oxazolone-induced colitis is easily distinguishable from most other forms of experimental colitis. First, it is a rapid onset inflammation that peaks within a few days after oxazolone administration and leads to wasting and bloody diarrhea, resulting in the death of the mouse, otherwise complete recovery. Second, oxazolone-induced colitis is marked by inflammation strictly limited to the distal half of the colon. At the microscopic level, the inflammation of oxazolone-induced colitis is relatively superficial and is characterized by ulceration, cellular exudates, and a mixed inflammatory infiltrate of lymphocytes, granulocytes, and eosinophils. Third, and perhaps most importantly, in oxazolone-induced colitis, the T cell response is accompanied by markedly elevated IL-4 production and prevented by the systemic co-administration of anti-IL4 antibody (15). Thus the oxazolone-induced colitis is a suitable model of colitis for evaluation of the CIITA effect on IL-4 production and Th2 inflammation in vivo that has features resembling the human disease, ulcerative colitis. As we expected, oxazolone-induced colonic inflammation was aggravated in CIITA-Tg mice (Fig. 3, 4), confirming that the CIITA expression in CD4+ T cells tends to bias CD4+ T cell response into Th2.
In summary, the results shown in current study, when considered in conjunction with our previous results (13), provide additional support for the view that the constitutive expression of CIITA in T cells inhibits Th1 differentiation and promote Th2 differentiation both in vitro and in vivo.
Figures and Tables
 | Fig. 1Overexpression of CIITA expression in T cells did not affect DSS-mediated colitis. Wild type (WT) and CIITA-transgenic (Tg) mice were treated with 2.5% DSS for 7 days and body weight was monitored every day. DSS significantly reduced body weight for 7 days; this decrease in body weight was not affected by CIITA expression in T cells. Each point represents average weight data. The standard errors are indicated. |
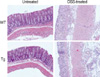 | Fig. 2Histopathological features of wild type and CIITA-transgenic mouse intestines showing similar inflammation in response to DSS treatment. Colonic samples were taken both from wild type (WT) and CIITA-transgenic (Tg) mice receiving either water (control) or DSS on day 7. Compared with that of control animals (left), colons of DSS-treated mice (right) shows complete destruction of epithelial architecture, with nearly complete loss of crypts, loss of epithelial integrity, and intense cellular inflammation in all layers. CIITA expression (right lower) does not affect morphological damage and colonic inflammation shown in these mice is comparable to that of wild type mice (right upper). (H&E stain, ×100). |
 | Fig. 3Overexpression of CIITA in T cells aggravated the development of oxazolone-induced colitis. (A) Weight changes of wild type (WT) and CIITA-transgenic (Tg) mice treated with 50% ethanol alone or oxazolone in 50% ethanol. Mice were initially presensitized with epicutaneous application of oxazolone, followed by intrarectal administration of 1% oxazolone. After initial reduction of the body weight in both oxazolone-treated groups, the WT mice showed rapid increase in the average body weight after day 2, compared with that of CIITA-transgenic (Tg) mice. Each point represents average weight data pooled from 9 or 15 mice. The standard errors are indicated. (B) Survival of wild type mice treated with oxazolone was similar to that of CIITA-transgenic mice treated with oxazolone. *, p<0.05; †, p<0.01 |
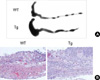 | Fig. 4Gross and microscopic features of intestines showing aggravated inflammation in CIITA-transgenic (Tg) mice compared with wild type (WT) mice in response to oxazolone. (A) Treatment with oxazolone induced significant shortening of the colon, which was more significant in CIITA-transgenic mice. (B) Histology of colonic samples taken from mice receiving oxazolone. Colon from oxazolone-treated CIITA-transgenic mice on day 3 after the challenge of oxazolone per rectum shows more severe inflammation characterized by epithelial ulceration, destruction of glands and inflammatory cell infiltration in whole layer of bowel wall (right). While in oxazolone treated wild type mice (left), the colon shows restricted inflammatory cell infiltration to mucosa. (H&E stain, ×100). |
 | Fig. 5Altered cytokine production from CD4+ T cells in CIITA-transgenic mice with oxazolone-induced colitis. CD4+ T cells were isolated from mesenteric lymph node of wild type (WT) and CIITA-transgenic (Tg) mice on day 3 after induction of oxazolone colitis and stimulated in vitro for 3 days with anti-CD3 antibody (2C11). The levels of cytokines secreted in culture supernatant were determined by ELISA. |
References
1. Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994. 180:1367–1374.

2. Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science. 1994. 265:106–109.
3. Chang CH, Flavell RA. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med. 1995. 181:765–767.

4. Dong Y, Rohn WM, Benveniste EN. IFN-γ regulation of the type IV class II transactivator promoter in astrocytes. J Immunol. 1999. 162:4731–4739.
5. Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002. 109:Suppl. S21–S33.

6. Xu Y, Wang L, Buttice G, Sengupta PK, Smith BD. Major histocompatibility class II transactivator (CIITA) mediates repression of collagen (COL1A2) transcription by interferonγ (IFN-γ). J Biol Chem. 2004. 279:41319–41332.
7. Nozell S, Ma Z, Wilson C, Shah R, Benveniste EN. Class II major histocompatibility complex transactivator (CIITA) inhibits matrix metalloproteinase-9 gene expression. J Biol Chem. 2004. 279:38577–38589.

8. Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000. 165:2511–2517.

9. Gourley TS, Chang CH. Cutting edge: the class II transactivator prevents activation-induced cell death by inhibiting fas ligand gene expression. J Immunol. 2001. 166:2917–2921.

10. Montani V, Shong M, Taniguchi SI, Suzuki K, Giuliani C, Napolitano G, Saito J, Saji M, Fiorentino B, Reimold AM, Singer DS, Kohn LD. Regulation of major histocompatibility class II gene expression in FRTL-5 thyrocytes: opposite effects of interferon and methimazole. Endocrinology. 1998. 139:290–302.

11. Kim WB, Kim TY, Park YJ, Koh JJ, Park DJ, Cho BY. Effect of class 2 transactivator on expression of thyroid transcription factor-1 in FRTL-5 cells. J Korean Soc Endocrinol. 2002. 17:48–56.
12. Gourley T, Roys S, Lukacs NW, Kunkel SL, Flavell RA, Chang CH. A novel role for the major histocompatibility complex class II transactivator CIITA in the repression of IL-4 production. Immunity. 1999. 10:377–386.

13. Park WS, Bae Y, Chung DH, Choi YL, Kim BK, Sung YC, Choi EY, Park SH, Jung KC. T cell expression of CIITA represses Th1 immunity. Int Immunol. 2004. 16:1355–1364.

14. Otten LA, Tacchini-Cottier F, Lohoff M, Annunziato F, Cosmi L, Scarpellino L, Louis J, Steimle V, Reith W, Acha-Orbea H. Deregulated MHC class II transactivator expression leads to a strong Th2 bias in CD4+ T lymphocytes. J Immunol. 2003. 170:1150–1157.
15. Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998. 188:1929–1939.

16. Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002. 17:629–638.

17. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98:694–702.

18. Gourley TS, Patel DR, Nickerson K, Hong SC, Chang CH. Aberrant expression of fas ligand in mice deficient for the MHC class II transactivator. J Immunol. 2002. 168:4414–4419.

19. Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo MC, Kunkel SL. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994. 145:1105–1113.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download