Abstract
The purpose of this study is to evaluate predictors of success of repeated injections of methotrexate in the single-dose regimen for the treatment of tubal ectopic pregnancy. All patients who had ectopic tubal pregnancy and were treated with a single dose regimen were retrospectively identified. 126 patients were treated with methotrexate. Among them, 39 patients were adequate for this study. 33 were treated with the 2nd dose and 27 were successfully cured. Additionally, 6 who were injected with the 3rd dose were all cured as well. Therefore, in our study, the success rate for the repeated injections of methotrexate was found to be 84.6% (33/39). The mean initial β-hCG level was significantly lower in patients who were successfully treated than in patients who failed (3915.3±3281.3 vs. 8379.7±2604.4 IU/mL, p<0.05). The success rate is 96% when the β-hCG level is less than 6,000 IU/mL and is 58% when β-hCG is greater than 6,000 IU/mL (OR=18.57, 95% CI 1.86-185.89). The initial β-hCG level is the only factor that has significant meaning as predictor of success of repeated injections of methotrexate in the single-dose regimen. Repeated injections of methotrexate may be particularly effective when the initial β-hCG level is below 6,000 IU/mL.
The use of methotrexate (MTX) for medical treatment of women with tubal ectopic pregnancy was first introduced in 1982, and has now come to be widely accepted. Additionally, the role of MTX has become more important as a consequence of the current widespread availability of the early diagnosis of ectopic pregnancy.
Initially, MTX has been used in a multi-dose regimen with citrovorum rescue factor (1, 2). It was then documented that ectopic pregnancy was successfully treated with a single intramuscular injection of MTX (3, 4). Success rates of the single-dose regimen in the treatment of ectopic pregnancy have been reported to range from approximately 75% to greater than 90% (4-6) with lower toxic side effects, fewer complications, and better acceptance by patients when compared to multi-dose regimens (7).
Although predictors of success of the single-dose regimen are widely known from various studies, the success predictors of repeated injections of MTX, due to the decline of β-hCG level less than 15% between days 4 and 7, have not yet been acknowledged. For this reason, when repeated injections are used, doctors and patients tend to fear failure, especially when β-hCG level increases markedly between days 4 and 7. This often leads to the decision of elective operation, rather than attempting the additional injections of MTX.
The purpose of this study is to evaluate predictors of success of repeated injections in the single-dose regimen for the treatment of tubal ectopic pregnancy.
Between January 2001 and December 2003, all patients who had tubal ectopic pregnancy and were treated with a single-dose regimen were retrospectively identified from hospital records of the Department of Obstetrics and Gynecology in the Korea University Medical Center. The diagnosis of tubal pregnancy was made using both transvaginal ultrasonography (TVU) and measurement of β-hCG level. Uterine curretage was performed only when clinically indicated. Ectopic pregnancy was diagnosed when adnexal mass or extrauterine tubal gestational sac without intrauterine gestation was observed with TVU and when patients had inappropriately rising β-hCG levels (slower doubling time after one to two consecutive β-hCG level determinations).
An operation was performed on hemodynamically unstable patients with signs and symptoms of rupture, as well as for patients who refused medical treatment with MTX and close follow-up. Patients were not excluded from MTX therapy by a selected initial β-hCG level or by the presence of fetal cardiac activity observed by TVU.
All patients were treated with a single IM dose (50 mg/m2) of MTX. Before MTX therapy, each patient was informed about the possible side effects of MTX or tubal rupture and intraabdominal hemorrhage, which would necessitate surgery. β-hCG levels were measured on days 4 and 7 after the injection. Patients received an injection of MTX if their β-hCG levels failed to decline by at least 15% between days 4 and 7. A successful response to MTX was defined as the resolution of the β-hCG level less than 10 IU/mL. Treatment failure was defined as the need to undergo surgical intervention for women with signs of presumed tubal rupture (i.e., hemodynamic instability, falling hematocrit level, or sonographic visualization of free fluid outside the pelvis) or for women for whom three injections of MTX failed to resolve the ectopic pregnancy.
All continuous variables are expressed as the mean±SD unless otherwise specified. T-test, chi-square test, and ANOVA were performed using SPSS software (SPSS 10.0, Inc, Chicago, IL, U.S.A.). p<0.05 was considered statistically significant.
During the study period, 126 patients were treated with MTX. Among these patients, 85 patients (73 of which were completely resolved and 12 of which underwent operation due to ectopic rupture) who were once injected with MTX were excluded. 2 patients who underwent operation on their requests after the first injection of MTX were excluded as well, making the remaining 39 patients the subjects of this study. Among the 39 patients, 33 were injected with the 2nd dose of MTX and 27 were cured. Of the 6 who were injected with the 3rd dose of MTX, all were cured. Therefore, the success rate for the repeated injections was found to be 84.6% (33/39) in our study (Fig. 1).
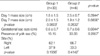
For the purpose of analysis, we classified the patients into two groups; patients whose ectopic pregnancies resolved after receiving repeated injections were classified as group 1 and patients who underwent operation due to ectopic rupture after repeated injections were classified as group 2. There were no statistical differences with age, gestational age, gravidity, history of ART, and symptoms such as lower abdominal pain and/or vaginal spotting between group 1 (n=33) and group 2 (n=6). Moreover, no statistical differences were found in the size of ectopic mass on days 1 and 7, gestational sac size, and the pregnancy site in sonographic findings between two groups (Table 1). Also, although the β-hCG levels of group 2 on days 1, 4, and 7 were all statistically high, there were no statistical differences in changes of β-hCG levels from days 1 to 4, from 4 to 7, and from 1 to 7 between the two groups (Table 2).
We then divided group 1 into groups 1-a and 1-b; group 1-a consisted of patients who showed a decrease in β-hCG level from day 4 to 7 and group 1-b consisted of patients who showed an increase in β-hCG level. When comparing the three groups, group 1-a, 1-b, and 2, a significantly higher β-hCG level was observed in group 2. We also found that the changes in β-hCG level from day 4 to 7 were significantly different among the groups, with a larger increase rate observed in group 1-b compared to group 2. On the other hand, the three groups did not show any difference in the increase rate of β-hCG level from day 1 to 4 and day 1 to 7. Also, interestingly, the resolution time between the two groups with similar β-hCG levels, group 1-a and 1-b, did not show any statistical difference (Table 3, Fig. 2).
Therefore, we suggest that the only significant success predictor of repeated injections is the initial β-hCG level. The success rate is 96% (26/27) when initial β-hCG level is less than 6,000 IU/mL and 58% (7/12), when greater than 6,000 IU/mL (OR=18.57, 95% CI 1.86-185.89).
Through many studies, initial β-hCG level, size of an ectopic gestational mass, the presence of fetal cardiac activity, the presence of free peritoneal fluid, and the presence of pelvic pain or vaginal spotting have been accepted as predictors of success for a single-dose regimen (6, 8-11). The initial β-hCG level is known to be higher in patients who required a second MTX injection, as compared to patients treated with one dose, and Potter et al. (8). reported that the OR 3.8 indicates the failure of repeated injections of MTX treatment. However, predictors of success of repeated injections are not well known. The small number of patients enrolled in most studies and the exclusion of candidates for repeated injections due to strict inclusion criteria, such as low β-hCG level or small ectopic mass size, as in the study by Stovall et al. (7) in which only 3.3% (4 of 120 patients) were injected with the 2nd dose MTX, may account for this. In our studies, we found that the initial β-hCG level was the only predictor of success for the repeated injections, particularly in the cases with initial β-hCG levels lower than 6,000 IU/mL.
It has to be noted that the early rise of β-hCG levels can be observed in 50-70% of cases subjected to this medical therapy, although it is not always associated with a clinically relevant persistence of trophoblastic tissue (7, 12-15). Similarly, in our study, the early rising of β-hCG level was observed in 79.5% (31/39) of cases, our rates being higher since the patients showing a decrease in β-hCG levels on day 4 were mostly resolved after one dose injection and, as a result, excluded from this group. It still must be clarified why an initial increase in β-hCG level can be observed before a complete resolution and why it is present only in specific patients. Some hypotheses have addressed the interpretation of this issue by Natale et al. (14): 1) The long half-life (36 hr) of β-hCG level must be considered for an initial lag time in serum β-hCG clearance. 2) It is possible, but not yet unequivocally demonstrated, that the initial response of the trophoblast to the cytotoxic effect of methotrexate is the release of additional β-hCG into the circulation. 3) MTX is a folic acid analogue, an antimetabolite that interferes with the synthesis of DNA by inhibiting the action of dihydrofolate reductase; it interrupts the synthesis of the purine nucleotide thymidilate and the amino acids serine and methionine. This results in a cytotoxic effect on trophoblastic tissue. It is possible that, although MTX is arresting mitosis in cytotrophoblasts, the syncytotrophoblastic mass still may be increasing and producing β-hCG (16). 4) The metabolism of folinic acid in different patients might influence the effect of MTX. This is supported by findings by Hajenius et al. (17), who observed that in two groups of patients treated with multiple-dose MTX, different protocols of folinic acid administration determined different patterns of β-hCG curves.
Additionally, it has been observed that the increase of β-hCG level from day 4 to 7 is greater in group 1-b than in group 2. This phenomenon can be partially explained by the lower mitotic activity of the trophoblastic cells in cases with smaller increases in β-hCG levels from day 4 to 7. Consequently, in these cases, the trophoblastic tissue could result in relative resistance to an antimitotic drug. Therefore, it would be hasty to conclude the treatment as a failure based only on the large increase rate in β-hCG level from day 4 to 7.
Natale et al. reported that at day 21 after the medical treatment, β-hCG levels are similar for all patients undergoing resolution; thus, when it occurs, the rapid rise of β-hCG levels is associated with a rapid decrease after day 3 (14). Accordingly, in this study, similar β-hCG levels in patients undergoing resolution were observed at day 42.
According to Gamzu et al., the initial size of the ectopic mass was thought not to be related to the success of the MTX treatment and that MTX treatment in tubal pregnancy was followed by an initial increase in the size of the ectopic mass; thus such enlargement of the ectopic mass should not be considered as a higher risk for failure of treatment (18). In our sonographic findings, there was no statistical difference in the initial size of ectopic mass between groups 1 and 2. Although the size of ectopic mass increased in group 1 and decreased in group 2 from day 1 to 7, such findings were not statistically significant. In addition, although the presence of yolk sac or the presence of fetal cardiac activity on TVS was considered to be a significant predictor of treatment failure (8), the presence of yolk sac was not statistically different between the two groups in our study. Regarding fetal cardiac activity, only one patient was recognized; the presence of fetal cardiac activity is known to be highly relevant to the high β-hCG level (10). It is most likely that such cases were not included in our study, since most patients would have failed on the 1st treatment of MTX before a second injection was taken into consideration.
In conclusion, the initial β-hCG level is the only significant predictor of success of repeated injections, particularly when the β-hCG level is below 6,000 IU/mL. Also, predicting treatment failure based strictly on a high increase of β-hCG level from day 4 to 7 may be a hasty judgment.
References
1. Ory SJ, Villanueva AL, Sand PK, Tamura RK. Conservative treatment of ectopic pregnancy with methotrexate. Am J Obstet Gynecol. 1986. 154:1299–1306.

2. Sauer MV, Gorrill MJ, Rodi IA, Yeko TR, Greenberg LH, Bustillo M, Gunning JE, Buster JE. Nonsurgical management of unruptured ectopic pregnancy: an extended clinical trial. Fertil Steril. 1987. 48:752–755.
3. Glock JL, Johnson JV, Brumsted JR. Efficacy and safety of single dose systemic methotrexate in the treatment of ectopic pregnancy. Fertil Steril. 1994. 62:716–721.
4. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991. 77:754–757.
5. Stika CS, Anderson L, Frederiksen MC. Single-dose methotrexate for the treatment of ectopic pregnancy: Northwestern Memorial Hospital three-year experience. Am J Obstet Gynecol. 1996. 174:1840–1848.

6. Lipscomb GH, Bran D, McCord ML, Portera JC, Ling FW. Analysis of three hundred fifteen ectopic pregnancies treated with single-dose methotrexate. Am J Obstet Gynecol. 1998. 178:1354–1358.

7. Stovall TG, Ling FW. Single-dose methotrexate: an expanded clinical trial. Am J Obstet Gynecol. 1993. 168:1759–1762.

8. Potter MB, Lepine LA, Jamieson DJ. Predictors of success with methotrexate treatment of tubal ectopic pregnancy at Grady Memorial Hospital. Am J Obstet Gynecol. 2003. 188:1192–1194.

9. Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999. 341:1974–1978.

10. Tawfiq A, Agameya AF, Claman P. Predictors of treatment failure for ectopic pregnancy treated with single-dose methotrexate. Fertil Steril. 2000. 74:877–880.

11. Erdem M, Erdem A, Arslan M, Oc A, Biberoglu K, Gursoy R. Single-dose methotrexate for the treatment of unruptured ectopic pregnancy. Arch Gynecol Obstet. 2004. 270:201–204.

12. Groutz A, Luxman D, Cohen JR, David MP. Rising beta-hCG titers following laparoscopic injection of methotrexate into unruptured, viable tubal pregnancies. Br J Obstet Gynaecol. 1993. 100:287–288.
13. Saraj AJ, Wilcox JG, Najmbadi S, Stein SM, Johnson MB, Paulson RJ. Resolution of hormonal markers of ectopic gestation: a randomized trial comparing single-dose intramuscular methotrexate with salpingostomy. Obstet Gynecol. 1998. 92:989–994.

14. Natale A, Busacca M, Candiani M, Gruft L, Izzo S, Felicetta I, Vignali M. Human chorionic gonadotropin patterns after a single dose of methotrexate for ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002. 100:227–230.

15. Natale A, Candiani M, Barbieri M, Calia C, Odorizzi MP, Busacca M. Pre- and post-treatment patterns of human chorionic gonadotropin for early detection of persistence after a single dose of methotrexate for ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2004. 117:87–92.

16. DeLoia JA, Stewart-Akers AM, Creinin MD. Effects of methotrexate on trophoblast proliferation and local immune responses. Hum Reprod. 1998. 13:1063–1069.

17. Hajenius PJ, Voigt RR, Engelsbel S, Mol BW, Hemrika DJ, Van der Veen F. Serum human chorionic gonadotropin clearance curves in patients with interstitial pregnancy treated with systemic methotrexate. Fertil Steril. 1996. 66:723–728.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download