Abstract
Japanese hop (Hop J) pollen has been considered as one of the major causative pollen allergens in the autumn season. We developed a new Hop J immunotherapy extract in collaboration with Allergopharma (Reinbeck, Germany) and investigated immunologic mechanisms during 3 yr immunotherapy. Twenty patients (13 asthma with rhinitis and 7 hay fever) were enrolled from Ajou University Hospital. Sera were collected before, 1 yr, and 3 yr after the immunotherapy. Changes of serum specific IgE, IgG1, and IgG4 levels to Hop J pollen extracts and serum IL-10, IL-12, TGFβ1 and soluble CD23 levels were monitored by ELISA. Skin reactivity and airway hyper-responsiveness to methacholine were improved during the study period. Specific IgG1 increased at 1 yr then decreased again at 3 yr, and specific IgG4 levels increased progressively (p<0.05, respectively), whereas total and specific IgE levels showed variable responses with no statistical significance. IL-10, TGF-β1 and soluble CD23 level began to decrease during first year and then further decreased during next two years with statistical significances. (p<0.05, respectively). In conclusion, these findings suggested the favorable effect of long term immunotherapy with Hop J pollen extracts can be explained by lowered IgE affinity and generation of specific IgG4, which may be mediated by IL-10 and TGF-β1.
Allergen immunotherapy has been widely applied to patients with asthma and rhinitis due to its favorable effects (1). Regarding the mechanism of allergen immunotherapy, recent studies have emphasized the role of immunomodulating cytokines such as IL-10, IL-12 and TGFβ1 to effect on T and B cells (2-4). Japanese hop (Hop J), a weed belonged to Cannabinaceae family is widespread in both rural and urban areas of Korea. It has been considered one of the major causative pollens of autumn pollinosis in this country (5). We reported a preliminary study showing a favorable clinical and immunologic effect after the one-year immunotherapy with Hop J pollen extracts (6). It is essential to extend the study period and observe the mechanism of Hop J pollen immunotherapy. To the best of our knowledge, this is the first study to observe changes of serum cytokines with clinical parameters during the three years' immunotherapy with Hop J pollen extracts.
Immunotherapy extract was prepared as described before (5). An adequate amount of pollens was collected in the Suwon area during the last 4 weeks of September 2003. The pollens were defatted, dried, and sent to Allergopharma Co., Germany for "Depo-Hop J" preparation, which was used for skin prick test and immunotherapy. Standardization process was carried out and presented as protein nitrogen units (PNU). The initial preparation was composed of three serial vials with strengths 1 (25 PNU), 2 (250 PNU), and 3 (2,500 PNU). For the maintenance treatment, vial 3 (2,500 PNU) was used, and was administered monthly for 3 yr. The initial dose was 0.1 mL, and the maximum tolerable dose was decided on an individual basis. The extracts were lyophilized, which was used for ELISA and IgE-immunoblot analysis.
Twenty patients (13 asthma with rhinitis and 7 hay fever) were enrolled. Asthma severity was classified as mild to moderate degrees based on the revised global initiative for asthma guideline (2004) and they had suffered from seasonal aggravation of asthmatic and rhinitis symptoms. All the patients had high serum-specific IgE antibody to Hop J by ELISA, as well as positive [>3+ (allergen to histamine ratio, A/H)] responses to skin prick tests. To exclude allergen exposure effect, Hop J immunotherapy was started in 1 month after the pollen season. Patients' sera were collected three times: before, one year and three years after the immunotherapy. All the patients gave their informed consent, and this study was approved by the ethical commitee of Ajou University Medical Center, Suwon, Korea.
Skin prick tests were performed three times: before, one year and three years after the immunotherapy and the skin reactivity was presented as A/H ratio. Degrees of airway hyperresponsiveness to methacholine were measured out of season before and every year using the method previously described (6).
Serum total IgE level was measured by Immuno-CAP system (Pharmacia, Sweden) according to the manufacturer's instructions. A change of specific IgE level to Hop J was determined by ELISA according to the previously described method (6). Briefly, microtiter plates (Costar, Corning, NY, U.S.A.) were first coated with 100 µL of Hop J pollen extract (1 µg/well) and left at 4℃ overnight. Each well was washed three times with 0.05% Tween-phosphate-buffered saline (PBS-T), and the remaining binding sites were blocked by incubation with 200 µL of 10% fetal bovine serum for 1 hr at room temperature. Each well was washed three times and then incubated for 1 hr at room temperature with 50 µL of either the patients' sera (1:5 dilution) or control sera from 60 patients who showed negative skin prick test responses to common inhalant allergens and the Hop J pollen. After the wells were washed three times with PBS-T, 100 µL of 1:1,000 (vol/vol) biotin-labeled goat anti-human IgE antibody (Sigma Co., St. Louis, MO, U.S.A.) was added to the wells and incubated for 1 hr at room temperature. The wells were then washed three times with PBS-T and incubated with 1:1,000 (vol/vol) streptavidin peroxidase (Sigma Co.) for 30 min before another washing step, which was followed by incubation with 100 µL of 3.3', 5.5'-tetramethylene benzidine in 0.05 M/L phosphate citrate buffer, pH 5.0, and 10 mL of 30% H2O2 in a phosphate citrate buffer for 10 min at room temperature. The reaction was stopped by the addition of 100 µL of 2 N sulfuric acid, and the absorbance was read at 450 nm by an automated reader.
Diluted patient serum or negative control serum (1:3 for soluble IgG1 and 1:100 for soluble IgG4 in diluent buffer; PBS containing 10% fetal bovine serum; 200 µL) was added to each well coated with Hop J pollen. After incubation for 1 hr at 25℃, the wells were washed three times with PBS-T. After they were washed three times with PBS-T, 100 µL of 1:1,000 (vol/vol) biotin-labeled goat anti-human IgG1 or IgG4 antibody (Sigma Co.) were added to the wells and incubated for 1 hr at room temperature. The wells were then washed three times with PSB-T and incubated with 1:1,000 (vol/vol) streptavidin peroxidase (Sigma Co.) for 30 min before another washing step, which was followed by incubation with 100 µL of 3.3', 5.5'-tetramethylene benzidine in 0.05 M phosphate citrate buffer, pH 5.0, and 10 mL of 30% H2O2 in a phosphate citrate buffer for 10 min at room temperature. The reaction was stopped by the addition of 100 µL of 2 N sulfuric acid, and the absorbance was read at 450 nm by an automated reader. The antibody titer was expressed as arbitrary unit (A.U.) absorbance values. The positive cut-off value was determined as mean +3 standard deviations (SD) of 60 controls. All the samples were run on the same day.
12% SDS-PAGE and IgG4-immunoblot analysis were performed under reducing conditions according to methods described previously (6). The Hop J pollen extracts were mixed with sample buffer (Tris-HCl 31 mM/L, 10% glycerol, 1% SDS, 0.0025%, bromophenol blue, 2.5% β-mercaptoethanol, pH 6.8) and heated in boiling water for 5 min. Then, standard marker (4 to 250 kDa; Novex, San Diego, CA, U.S.A.) and the Hop J pollen extracts were loaded to 12% Tris-glycine gel (Novex) for the separation of the antigens. Electrophoresis was performed with a Bio-Rad protein Mini cell for 90 min at 125 V. The gel was fixed and stained with Coomassie brilliant blue. For immunoblotting, the proteins were transferred onto a polyvinylidene difluoride membrane (PVDF) (Millipore Co., Bedford, MA, U.S.A.) in transfer buffer (Tris-base 25 mM/L, glycine 193 mM/L and methanol 20%) with a Bio-Rad transfer apparatus set at 200 mA for 90 min. The blotted PVDF membrane was sliced into 4 mm widths. The pieces of the membrane were blocked by using 5% skim milk in Tris-buffered saline (TBS)-Tween (TBST) for 1 hr to block nonspecific binding. Each membrane was then incubated overnight at 4℃ with the patient or control sera that had been diluted 1:10 v/v with 5% skim milk TBST. After washing, the membranes were incubated with anti-human IgG4 conjugated with biotin and streptavidine-alkaline phosphastase (1:1,000 diluted, Sigma Co.) for one hour at room temperature. After washing with TBST, the membrane was developed by using BCIP/NBT alkaline phosphatase substrate (Sigma Co.).
To observe the changes of cell marker and cytokines, the levels of IL-10, soluble CD23 (sCD23), IL-12 and TGF-β1 were measured by ELISA kits (Benger Med Systems, Vienna, Austria) according to the manufacturer's guidance. The changes between years were presented as the ratio between two values.
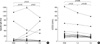
Fig. 1A shows the changes of skin reactivity to Hop J. It decreased after 1 yr of immunotherapy (p=0.019), however further decrease was not noted from 1 to 3 yr (p=0.86). Airway hyperresponsiveness to methacholine tended to improve at 1 yr (p=0.21) and improved further during next 2 yr (p=0.05) as shown in Fig. 1B.
Fig. 2 shows the changes of serum specific IgE to Hop J pollen extracts and sCD23 levels during 3 yr study period. There were no significant changes in either total (data not shown) or specific IgE antibody levels during the 3-yr of immunotherapy (p>0.05, respectively), however sCD23 level began to decrease during the first year and significantly decreased during next two years. (p=0.003)
Fig. 3 shows the changes of serum specific IgG1 (Fig. 3A) and IgG4 (Fig. 3B) antibodies to Hop J pollen extracts during 3 yr immunotherapy. Specific IgG1 levels increased significantly for first year with statistical significance (p<0.001), and then decreased significantly during next two years (p=0.0039). However, serum specific IgG4 levels began to increase remarkably during the first year (p<0.0001) and increased further until 3 yr with high statistical significance (p<0.001, Fig. 3B).
Fig. 4 shows the comparison of IgG4-binding components within the Hop J pollen extracts using the sera of seven individuals showing remarkable increase of serum specific IgG4 level during the study period. Five components (94, 78, 22, 13, 7 kDa) showing remarkable increase of IgG4 binding intensity were generated as shown in Fig. 4B.
Fig. 5 shows the changes of serum IL-10 (Fig. 5A) and TGF-β1 (Fig. 5B) levels during 3 yr immunotherapy. There were no significant changes in serum IL-10 during the first year (p=0.55), but decreased significantly during next two years with statistical significance (p=0.05). TGF-β1 levels began to decrease during the first year and decreased further during next two years of immunotherapy with statistical significance (p=0.01). There were no significant changes in serum IL-12 levels during 3 yr study period (data not shown).
Large-scale studies evaluating the efficacy of subcutaneous immunotherapy with inhalant allergens have been undertaken in patients with pollen asthma. Double-blind placebo controlled studies using standardized vaccines have shown that immunotherapy has a beneficial effect on bronchial symptoms and/or decreases the need for asthma medications in both pollen and house dust mite asthma (1, 7, 8).
Japanese hop pollen has been reported as one of the major allergenic pollens in Far Eastern Asian countries (5) and there has been no clinical trial looking at the effect of long term allergen immunotherapy in the sensitized patients. This is the first study to look at immunologic changes after long-term Hop J pollen immunotherapy in the sensitized patients, in which the study subjects were different from those enrolled in our previous study (6). Pollens were collected from our region and sent to Allergopharma Co, German. Alum precipitated immunotherapy extract was prepared according to it's standardized protocol. In this study, after 3 yr of immunotherapy, skin reactivity to allergen decreased earlier while further decrease was not noted from 1 to 3 yr. The changes in both skin reactivity and serum specific IgE levels to allergen were not significantly decreased over 3 yr of immunotherapy. However, airway hyperresponsiveness to methacholine improved in some patients from first one year and significantly improved during next two years. The change of sCD23 levels was on the whole the same as that of airway hyperresponsiveness, it decreased significantly during next two years. CD23, a differentiation marker of B cells and low affinity IgE Fc receptor on antigen presenting cells, is continuously cleaved by autolysis into soluble fragments called sCD23 that have been found in patients with allergic diseases (10). Others have reported a reduction of sCD23 levels during allergen immunotherapy in allergic rhinitis and pollinosis (11, 12, 13). These findings suggest that both serum sCD23 levels and airway hyperresponsiveness to methacholine can be improved significantly after the Hop J pollen immunotherapy.
These findings suggest that both skin reactivity and airway hyperresponsiveness to methacholine can be improved significantly after the Hop J pollen immunotherapy.
Regarding the mechanism of allergen immunotherapy, earlier studies were focused on the changes of circulating antibodies and effector cells, serum specific IgE antibody levels were decreased, but specific IgG levels were increased (7, 8, 13). However, recent studies, which demonstrated an immunomodulating effect from Th2 to Th1 responses, cytokine regulation of the immune responses, and specific inhibition or ablation of immune responses by means of tolerance induction (4, 5, 14, 15), have reinforced the importance and value of allergen immunotherapy. In this study, the consistent findings for increased levels of serum specific IgG1 and IgG4 antibodies were confirmed, however specific IgG1 levels were increased during first year and then decreased again, while specific IgG4 increased progressively during the 3 yr study period. These findings suggested that improvement in clinical parameters after three years' immunotherapy may be derived from enhanced production of serum specific IgG4 and lowered IgE affinity, not from the effect of specific IgG1 or specific IgE. Generation of five IgG4 components were noted from first year and their intensities were increased up to third year.
There have been several reports demonstrating the involvement of IL-10 and TGF-β1 to induce immunomodulating effects after the allergen immunotherapy (2-4, 15). These two cytokines could induce T-cell and B-cell suppression; IL-10 inhibits IgE and enhances IgG4 production in pollen allergy studies (16, 17) and also it is a general inhibitor of proliferative and cytokine responses in T cells with TGF-β1. TGF-β is a pleiotropic cytokine known to affect T cell proliferation, differentiation, apoptosis, antigen presentation, effector functions of macrophages, the expression of MHC class II15, 16 and I. Allergen immunotherapy could also affect IgE binding on B cell (10, 12, 18). These findings suggest that allergen immunotherapy could induce TGF-β and IL-10 productions from T cells, which result in reduction of specific IgE with lowered affinity, but increased production of specific IgG4 with high affinity, as suggested by a recent review (2). In this study, serum IL-10 and TGF-β1 levels tended to decrease from first year and further decreased until third year. The correlation data showed that the greater decrease in TGF-β1, the more decrease in sCD23, but higher increase of specific IgG4 antibody (data not shown). Although this is a limited study to explain these findings, the decreased level of IL-10 and TGF-β1 may be explained by compartmentalization of these two cytokines into major target tissues to present immunomodulating effect there. In conclusion, we confirmed favorable effects in clinical parameters after the long-term immunotherapy with Hop J pollen extracts, which could be explained by increased production of specific IgG4 antibodies and decreased IgE binding affinities in associations with TGF-β1 and IL-10.
Figures and Tables
Fig. 1
Changes of skin reactivity (A) and PC20 methacholine (B) level during the 3 yr immunotherapy.

Fig. 3
Changes of serum specific IgG1 (A) and specific IgG4 (B) level during the 3 yr' immunotherapy.

References
1. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic disease. J Allergy Clin Immunol. 1998. 102:558–562.
3. Jutel M, Akdis M, Budak F, Casaulta CA, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003. 33:1205–1214.

4. Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, Staple SQ, Aalberse RC, Till SJ, Durham SR. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004. 172:3252–3259.

5. Park HS, Nahm DH, Suh CH, Lee SM, Choi SY, Jung KS, Lee SY, Park K. Evidence of Hop Japanese pollinosis in Korea: IgE sensitization and identification of allergenic components. J Allergy Clin Immunol. 1997. 100:475–479.

6. Park HS, Nahm DH, Kim HY, Suh YJ, Cho JW, Kim SS, Lee SK, Jung KS. Clinical and immunologic changes after allergen immunotherapy with Hop Japanese pollen. Ann Allergy Asthma Immunol. 2001. 86:444–448.

7. Bonifazi F, Bilo MB. Efficacy of specific immunotherapy in allergic asthma: myth or reality? Allergy. 1997. 52:698–710.

8. Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J Allergy Clin Immunol. 1998. 102:157–164.

9. Taicopoulos A, Joseph M. The role of CD23 in allergic disease. Clin Exp Allergy. 2000. 30:602–605.
10. Tanaka A, Ohashi Y, Nakai Y. Decrease of serum levels of soluble CD23 during immunotherapy in patients with perennial allergic rhinitis. Ann Otol Rhinol Laryngol. 1999. 108:193–200.

11. Boznanski A, Willak JE, Domanasiewicz M, Pirogowicz I, Widerska A. Evaluation of soluble components of receptors with weak affinity for IgE (sCD23) in children with pollinosis given specific immunotherapy. Pneumonol Alergol Pol. 1996. 64:386–391.
12. Van Neerven RJ, Arvidsson M, Ipsen H, Sparholt SH, Rak S, Würtzen PA. A double blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation. Clin Exp Allergy. 2004. 34:420–428.
13. Jenmalm MC, Björkstén B, Macaubas C, Holt BJ, Smallacombe TB, Holt PG. Allergen-induced cytokine secretion in relation to atopic symptoms and immunoglobulin E and immunoglobulin G subclass antibody responses. Pediatr Allergy Immunol. 1999. 10:168–177.

14. Malling HJ. Allergen-specific immunotherapy: present state and directions for the future. Allergy. 1999. 54:30–33.
15. Wilcock LK, Francis JN, Durham SR. Aluminium hydroxide down-regulates T helper 2 responses by allergen-stimulated human peripheral blood mononuclear cells. Clin Exp Allergy. 2004. 34:1373–1378.

16. Punneonen JR, Waal MR, Van VP, Gauchat JF, Vries JE. IL-10 and viral IL-10 prevent IL-4 induced IgE synthesis by inhibiting the accessory cell function of monocytes. J Immunol. 1993. 151:1280–1289.
17. Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. The role of IL-10 in specific immunotherapy. J Clin Invest. 1998. 102:98–106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download