Abstract
β-glucan is a polysaccharide in the form of fiber and the main element of fiber in grains such as barley, oats, yeast and mushrooms. Many studies have examined the efficacy of β-glucan in terms of the lipid lowering effects, blood sugar reduction, weight reduction, immune modulator, and anticarcinogenic effect. However, there is no comprehensive review article on the biomedical issues regarding β-glucan. The authors searched for systematic reviews and clinical experiments for each relevant topic and reviewed the biomedical effects of β-glucan, for the purpose of developing research strategies for the future.
β-glucan is a polysaccharide in the form of fiber that is found in baker's yeast, oats and barley fiber as well as medicinal mushrooms. β-glucan is present in natural yeast and mushrooms mainly as β-1,3-glucan or β-1,6-glucan, and as β-1,3-glucan and β-1,4-glucan in oats and barley (Fig. 1).
The commercial β-glucan extract is usually produced from yeast such as baker's yeast or Saccharomyces cerevisiae (1). In Korea, there are many β-glucan products available on the market, which are generally extracted from mushrooms, such as Phellinus linteus or Sparassis crispa.
β-glucan has been evaluated with regard to its various effects, including its immune modulating effects, anticarcinogenic effects, lipid lowering effects, as well as its ability to reduce the blood sugar levels and weight. It is a type of dietary fiber that is defined as indigestible plants carbohydrate.
Until recently, dietary fiber was divided into two types: hydrosoluble and insoluble. Hydrosoluble fibers lower the blood cholesterol level and delay the absorption of sugar, while insoluble fibers increase the volume of the stool. However, this categorization does not accurately represent the effects of all fibers. For example, oat bran lowers the cholesterol levels but wheat bran, which is also a hydrosoluble fiber, does not (2). In addition, cornstarch, which is a hydrosoluble fiber, does not lower the cholesterol levels. Moreover, while a large number of insoluble fibers increase the quantity of stools, cellulose does not. However, although their categorization suggests that they should not, some hydrosoluble fibers, such as oat bran and psyllium, increase the quantity of stools.
The failure of the categories, hydrosoluble and insoluble, to accurately classify the effects of various fibers has resulted in their abandonment by the U.S. National Academy of Sciences Panel on the Definition of Dietary Fiber. As a prelude to a more accurate categorization, they have decided to examine the physiological effects of each fiber separately. The panel determined that the effects of dietary fiber depended on the viscosity and fermentability (3), and offered the following categories: 1) total fiber, which is the sum of dietary fiber and functional fiber, 2) functional fibers, which are physiologically beneficial nondigestible carbohydrates. These categories are quite broad and need to be refined considerably if they are to be of any use in clinical settings. The panel also determined that the recommended daily intake of fiber is 38 g and 25 g for adult men and women or adolescents, respectively (4). The aim of this study was to determine future research directions.
The effects of β-glucan in yeast or grain were examined in various clinical settings. The relevant literature was searched in Pubmed (1966-November to 2005). The keywords used in the search were 'β-glucan OR oat OR barley.' The clinical situations were classified as follows: cholesterol reduction, blood sugar control, weight control, infection, and cancer treatment. For each area, a search was made for systematic reviews and clinical reports.
Among the menus of Pubmed 'Find Systematic Reviews' in 'Clinical Queries' was used to find the systematic reviews, and a randomized controlled trial [pt] was used to locate the randomized controlled studies. The following keywords were also used: 'cholesterol' for cholesterol, 'glucose OR diabetes mellitus' for sugar control, 'weight OR obesity' for weight control, 'infect* OR inflama*' for infection and 'neoplasm OR cancer*' for anticarcinogenic effect. The suitability was determined based on the abstracts and titles of the theses. However, many of the selected studies were not randomized even though some of them were controlled. Therefore, some clinical experiments, all randomized controlled studies and systematic reviews relevant to the corresponding theme were included.
The substances used as immune modulator drugs via injection in clinical and in vitro experiments were sizofiran (SPG, Schizophyllum commune a β-1, 3 glucan extract, obtained from cultured Schizophyllum commune fries), Lentinan (Lentinus edodes β-1, 3 glucan extract), PGG-glucan (poly-[1,6]-B-D-glucopyranosyl-1,3-B-D-glucopyranose), etc. These substances act in the following manner: 1) promote the secretion of cytokines such as TNF-α and IL-1-β , which bind to -glucan receptors in macrophage and neutrophils that form part of the nonspecific immune system, 2) suppress the secretion of superoxide anion and hydrogen peroxide, and 3) increase the activity of natural killer and lymphokine-activated killer (LAK) cells, which contribute to their germicidal and anticarcinogenic effects (5-10).
Recently, there has been increasing interest in the effect of hydrosoluble dietary fiber, on lowering the blood cholesterol concentration. There are various mechanisms by which cholesterol is reduced by dietary fiber: binding to bile, viscosity in the small intestine, suppression of glucose absorption, and increased production of short-chain fatty acids (11-15).
It is unclear if these characteristics are also applicable to most types of dietary fiber. According to meta-analysis, a daily intake of 2-10 g of hydrosoluble fiber, such as oat bran, pectin, psyllium and guar gum, reduces the total cholesterol and LDL cholesterol level by a small but significant amount (reduction by 2 mg/dL per 1 g of cellulose) but does not affect the HDL cholesterol or trigyceride level (16). However, dietary fibers differ in viscosity and fermentability, and their effects depend on these properties (3). Therefore, it is essential to determine the function of all dietary fibers individually.
There have been few systematic reviews on the effect of β-glucan on the blood lipids. However, there are two systematic reviews on the effect of oats on cholesterol. Ripsin et al. examined 10 randomized controlled studies on the effect of meals containing oats on reducing the cholesterol level in humans. The quantity of hydrosoluble fiber ranged from 1.1 to 7.6 g, and the meals were fed for periods ranging from 18 days to 12 weeks. When the results of these studies were metaanalyzed, the cholesterol level was reduced by 5.9 mg/dL (95% CI 3.3-8.4 mg/dL). The effect was high when the initial cholesterol level was more than 229 mg/dL and when the quantity of hydrosoluble fiber was >3 g (17). In 1999, Brown et al. reported the results of meta-analysis on the effect of cholesterol reduction when hydrosoluble fibers such as pectin, oat bran, guar gum and psyllium were administered. They showed that the cholesterol level decreased by 1.73 mg/dL per gram of hydrosoluble fiber when 2-10 g of hydrosoluble fiber was administered, which is not very high but significant. The results also showed that oats, pectin and psyllium had a similar effect on the blood lipids, and did not affect the triglyceride or HDL cholesterol levels. In addition, the results did not show a difference between each research design, treatment period, the quantity of dietary fat (16).
Baarten et al. reported that oat bran reduces the cholesterol level primarily because of its β-glucan content. They mixed 7.2 g of oat gum (5.8 g in β-glucan and 70 g in oat bran) with water and administered it to 20 hypercholesterolemic patients for four weeks. They reported that the cholesterol level decreased by 9% in the intervention group, while there was no difference in the placebo (maltodextrin) group. In addition, the high-density lipoprotein (HDL) and triglyceride levels in the two groups were similar (18). In 1997, the U.S. Food and Drug Administration (FDA) acknowledged that hydrosoluble fiber obtained from oat (oat bran, oatmeal and oat flour) can reduce the risk of cardiac disease (Table 1). The FDA reported that more than 3 g of hydrosoluble fiber from oats per day are needed to achieve significant reductions in cholesterol. Most studies on β-glucan in oats produced positive results but some studies reported no effect (19-22). This inconsistency can be explained in several ways: amount of β-glucan intake, the type of diet or supplements, the molecular size of β-glucan, the baseline cholesterol level, dose-response and long-term effect.
The variation in the effect might result from the differences in intake. For example, the minimum effective intake of 3 g a day might not be sufficient to yield significant results. Indeed, Lovegrove et al. reported that there was no difference in the cholesterol level when 62 patients with moderate hypercholesterolemia were fed either 20 g of oat bran concentrate (3 g of β-glucan) or wheat bran together with low-fat milk or yogurt for eight weeks. They attributed this to the β-glucan intake being too low (19). However, Karmally et al. reported that the total cholesterol level fell significantly by 10.9±21.6 mg/dL (4.5%) when 152 Hispanic people were fed either oat cereal (3 g of β-glucan per day) or corn cereal for six weeks (23). This shows that the intake alone cannot explain the degree to which β-glucan is effective in reducing the cholesterol level.
It was suggested that cooking or the composition of food can explain the differences in the efficacy of β-glucan. Kerckhoffs et al. fed 48 mild hyperlipidemia patients β-glucan containing bread and biscuits (5.9 g of β-glucan) or wheat bread and biscuit (fiber only, no β-glucan) for two weeks and compared the results. They reported that there was no significant difference in the cholesterol level. In contrast, when the same quantity of β-glucan was administered to the same group in the form of juice, the LDL cholesterol level was reduced significantly compared with the control group. They attributed this difference to the composition of food or the process of food preparation (24). Indeed, many studies in which β-glucan had been administered in bread did not report any reduction in the cholesterol level. Torronen et al. reported no difference in the lipid level when oat bran bread (11.2 g of β-glucan) was fed to patients with mild or moderate hypercholesterolemia for eight weeks (20), and Leadbetter et al. also reported no significant difference in the cholesterol level in 40 hyperlipidemic patients given bread prepared with 30, 60 or 90 g of oat fiber was (25). However, some studies report that feeding hyperlipidemic patients β-glucan in bread was effective (26).
Differences in the molecular size of β-glucan have been reported to have an effect. The effect of β-glucan on cholesterol reduction can be explained in terms of the reduction of bile reabsorption or the increase in viscosity in the small intestine. If the molecular size is small, the viscosity decreases and the effectiveness of β-glucan is reduced. Torronen et al. (20) and Beer et al. (21) explained the ineffectiveness of the intake of β-glucan by the small molecular size of the sample ingested. The molecular size of β-glucan used by Torronen et al. was relatively small (370,000) and that used by Beer et al. was 1,000,000. The molecular size of β-glucan used by Braaten et al. was 1,200,000, in which β-glucan was effective in reducing the cholesterol level (18). This suggests that the molecular size should be at least 1,200,000 for β-glucan to be effective. However, the molecular size of β-glucan used in Kerckhoffs et al. study was less than 100,000 and was found to be effective (24). Therefore, this mechanism cannot explain all the results either.
The variation in the baseline cholesterol level of subjects in clinical experiments may also explain some of the differences in the results. In meta-analysis by Ripsin et al. on the effect of oat products on reducing the cholesterol level, the baseline cholesterol level was found to be an important factor in determining the extent cholesterol reduction (17). However, Brown et al. reported that there was virtually no relationship between the baseline cholesterol level and the reduction in cholesterol (16). Among the three clinical experiments on patients without hyperlipidemia, two produced effective results but one did not. Beer et al. reported no significant difference in the total cholesterol and LDL cholesterol levels of 14 randomly selected young men who ingested oat gum (9 g of β-glucan per day) for 14 days (25). Robitaille et al. reported no significant change in the cholesterol level when 34 menopausal women were fed oat bran muffins for 4 weeks (22). In contrast, Reyna et al. reported that the lipid level in 16 well-controlled Type 2 diabetic patients was improved more by a low-calorie diet (containing oat β-glucan instead of fat) than by the diet recommended by the American Diabetes Association (27).
As suggested by these results, most studies have found oats to be effective in reducing the cholesterol level while some studies have reported the opposite. This inconsistency cannot be explained by any single factor.
There are a number of other controversial issues regarding the effect of oats on cholesterol reduction. One is the dose-response. Meta-analysis by Ripsin et al. reported a nonlinear dose-response in all fibers, including oats (17). 10 g and 8 g of dietary fiber was a significant cutoff point for cholesterol and LDL cholesterol, respectively. Davison et al. reported that when 156 hyperlipidemia patients were fed 28, 56 or 84 g of oatmeal or oat bran for six weeks, a significant reduction in the LDL cholesterol level was observed only in the groups fed 84 g of oatmeal. The level of reduction in those given 28 g, 56 g and 84 g of oat bran were 10.1%, 15.9% and 11.5%, respectively. There appeared to be a general trend with dose but no obvious dose-response relationship (28).
Another controversial issue is the long-term effects. Uusitupa et al. fed 36 mild or moderate hypercholesterolemic patients either oat (10.3 g of β-glucan) or wheat bran for eight weeks and found that the total cholesterol and LDL cholesterol levels were reduced in the oat bran group in the first four weeks. However, there was no significant change observed at the end of eight weeks. The cholesterol level was reduced by 6% in the first four weeks, which is a significant reduction, but by the end of the eight weeks the difference was only 3%, which is not statistically significant (29, 30).
There were four articles on the effects of a barley extract or barley diet on the lipid level. Of them, three reported a reduction in the cholesterol level, while one reported that it was not. McIntosh et al. reported that when 21 patients with light hypercholesterolemia ingested 8 g of β-glucan through a barley diet (170 g/day) for four weeks, the total cholesterol and LDL cholesterol levels was reduced by 6% and 7%, respectively, compared with the same amount of wheat food (31). Lupton et al. reported that 79 hyperlipidemic patients fed meals supplemented with 3 g of barley oil extract or 30 g of barley wheat flour showed a decrease in the LDL cholesterol level by 6.5% (barley wheat flour)-9.2% (barley oil extract) than those given 20 g cellulose (32). Behall et al. examined 18 patients with moderate hypercholesterolemia. They showed that three groups fed different amounts of barley showed a linear reduction in the total cholesterol level of 14%, 17% and 20%, respectively, compared with that of the control group (33). However, Keogh et al. divided 18 patients with mild hyperlipidemia into two groups and gave them β-glucan extracted from barley (8.1-11.9 g per day) or 6.5-9.2 g of glucose with the same number of calories over a four-week period. However, they did not observe any difference in the total cholesterol level (34).
Because yeast also contains a significant amount of β-glucan, it can reduce the cholesterol level. However, in contrast to barley and oats, β-glucan in yeast is likely to be less effective due to its viscosity and solubility. There has been one study on the effect of yeast β-glucan on reducing the cholesterol level. Nicolosi et al. gave 15 g/day of yeast β-glucan in orange juice to 15 hypercholesterolemic patients for eight weeks and stopped the feeding for the following four weeks. The total cholesterol and LDL cholesterol levels decreased by 8% in the first eight weeks but the HDL cholesterol level increased by 16% by the end of 12 weeks. However, this clinical experiment was carried out without a control group, so it is difficult to determine the validity of the result (35). As mentioned above, yeast β-glucan has a low viscosity and hydrosolubility. Therefore, if the results of the clinical experiment are accepted, the effect is not likely to be caused by an increase in viscosity in the small intestine, and should be explained by a different mechanism, such as an increase in the production of short-chain fatty acids or decrease in the blood insulin concentration.
There is growing interest in the relationship between dietary fiber and diabetes. In general, if diabetic patients increase their intake of fiber, sugar absorption can be delayed and the concentration of insulin will fall. For this reason, diabetic patients are recommended to take 25-50 g of fiber per day (36). In addition, grains such as oats and barley generally have a low glycemic index, so they help to improve the glycometabolism. Jennie et al. compared the results of 14 randomized controlled trials that compared the effect of a diet with a low blood sugar index with other usual diets on diabetic patients. They reported that the HbA1C concentration decreased by 0.43% (95% CI 0.13-0.73) in the groups fed a diet with a low blood sugar index. This effect is comparable to that of acarbose and insulin lispro regarding the reduction of the postprandial blood sugar level (37). In general, grains, fruit, and vegetables are recommended because they contain many vitamins, minerals and fiber, which promote health. However, the American Diabetes Association has not yet made a judgment as to whether a higher intake of fiber is helpful in controlling sugar, in either people with diabetes or in the general population (38).
There have been many studies on whether β-glucan in oats or barley is helpful for controlling blood sugar in either people with diabetes or the general population. Most studies examined diabetic patients and reported relatively positive results. Tapola et al. fed oats, wheat flour and glucose to 12 diabetic patients and compared their blood sugar curves. The oat group showed a significantly smaller area below the postprandial blood sugar curve (39). Reyna et al. reported the results of well-controlled Type 2 diabetic patients fed a diet recommended by the American Diabetes Association and a low-calorie diet that contained β-glucan (oat extract instead of fat) for four weeks. They found that the β-glucan diet group showed greater improvement in the HbA1C level (27). Jenkins et al. fed 17 volunteer Type 2 diabetics with 50 g of white bread, commercial oatmeal cereal (4.4 g% β-glucan), β-glucan reinforced morning cereal (8.1 g% β-glucan) or a β-glucan reinforced bar (6.5 g% β-glucan) and measured their blood sugar level. They showed that the blood sugar of those subjects who ate the reinforced morning cereal and reinforced bar was lower than in those who ate the oatmeal cereal and white bread (40). Pick et al. gave 80 Type 2 diabetics an oat bran extract or white bread with the same number of calories for 12 weeks and found significant differences in the area below the average blood sugar curve and the insulin peak (41). They suggested that oat β-glucan fed to diabetics has positive effects on blood sugar, HbA1C, insulin, etc. and recommended an oat or barley diet for diabetic patients.
Several clinical experiments have examined the differences in the blood sugar level in conditions other than diabetes. However, the blood sugar was not their primary concern. Therefore, these studies may be problematic with respect to the sample size and duration. Moreover, it may be difficult to identify differences in the blood sugar level with fiber replacement because of rapid insulin-sugar response in health subjects (42). There were two randomized controlled studies that fed β-glucan to hyperlipidemic patients, both of which reported no difference in the blood glucose level of the sugar control through a β-glucan diet (31, 34).
A number of studies have examined the differences in the glycometabolism resulting from feeding a β-glucan diet to the general population. Bourdon et al. reported that when β-glucan-reinforced pasta and wheat pasta were fed to 11 healthy adults, the level of insulin secretion slowed and the increase in the blood sugar level was low (15). Li et al. reported that there was no difference in blood sugar in 10 volunteer women fed a standard diet and barley diet for four weeks (43). Lovergrove et al. reported no difference in the fasting blood sugar level and insulin secretion when oat bran concentrate or wheat bran was fed to 62 healthy adults over an eight week period (19). Juntunen et al. fed 20 adults with normal glucose tolerance with rye bread, wheat bread, rye bread containing oat extract, wheat pasta or white wheat bread and observed them for eight hours. The results showed that the blood sugar curve was similar. However, the insulin concentration was lowest in those fed with the bread containing the oat extract (44). Overall, these reports show that barley, oat, etc. does not have any significant effect on the blood sugar in people whose glucose tolerance is normal.
Another key issue is the effect on the amount of dietary fiber intake containing β-glucan. According to the observational studies, the energy intake increases with decreasing fiber intake (45) and the obesity rate is low in those whose food intake contains an adequate amount of fiber (46). Moreover, an inverse correlation has been reported between the fiber intake and weight (47) as well as with the body mass index. The Nurse's Health Study found that the amount of weight gain is lower in those whose diet contains an adequate amount of fiber (48). Most observational studies reported that the intake of fiber reduces the level of weight gain or the risk of obesity. However, several intervention studies did not report a clear conclusion. At best, there are reports showing that additional weight loss is achieved through the addition of fiber to a low-calorie diet (5.8 kg vs. 8.0 kg) (49) and that the weight decreases with increasing amount of carbohydrate in the diet (50). According to Howarth et al., the effect of dietary fiber intake on weight loss is greater in obese people, and an additional intake of 14 g of dietary fiber reduced the energy intake by 10% and reduced the weight by 1.9 kg over a four-month period (51). In general, the effect of fiber on weight control is affected by the blood sugar level, which is ultimately related to hunger, insulin secretion, gastric emptying time and the intestinal hormones response (42). However, because most clinical experiments do not distinguish between the different types of fiber, it is unclear if a specific fiber, β-glucan in particular, is more or less effective in reducing weight.
Some clinical experiments examined the effect of β-glucan on body weight. However, because the body weight was not the primary concern of these studies, they did not provide specific information on weight reduction. Two studies on diabetic patients reported that the supplementation of β-glucan through oats did not have any significant effect on the body weight (17, 41). In another study, no significant effect on weight was observed in 68 hyperlipidemic patients given a high fiber ( β-glucan) diet compared with a control group (40). However, these clinical experiments focused on the changes in blood sugar or blood lipid. Therefore, the long duration for observing weight change could be a limitation. The inconsistent ground data on the effect of dietary or supplementary β-glucan on weight highlights the need for further research.
Several laboratory and animals experiment have shown that yeast β-glucan has a nonspecific beneficial effect on the immune system (1), which may facilitate the prevention of infection or affect the progress of infections already contracted. Four studies examined whether or not feeding yeast β-glucan reduces the risk of a postoperative infection. Babineau et al. published two randomized controlled studies on the risk of postoperative infections in patients who had undergone thoracic or abdominal surgery. In the two studies, PGG-glucan was fed to 67 and 34 patients who had undergone thoracic and abdominal surgery, respectively, and reported a significantly lower risk of postoperative infections (52, 53). The Betafectin Gastrointestinal Study Group administered 0.5 mg/kg and 1.0 mg/kg of PGGβ-glucan and a placebo to 1,249 high-risk patients who had undergone gastrointestinal surgery and reported no difference in serious infections but showed that the risk of serious infection and death decreased by 39% in cases other than large intestine operations. However, the clinical experiment was stopped early because there were more frequent abnormal reactions in the PGGβ-glucan administered group (54). Hamano et al. reported that, when lentinan or a placebo was administered randomly to 25 patients who had undergone cardiopulmonary bypass surgery, the intervention group easily recovered from the lymphocyte hypofunction that had been observed after surgery (55). It was also reported that the frequencies of sepsis and death decreased significantly when a placebo or β-glucan was administered to 41 patients who had incurred severe damage but who had not undergone surgery (56).
There has been little randomized research on whether β-glucan is effective against infections. However, one study reported a significant reduction in the CD4 level when didanosine and lentinan were administered together to 107 patients who were HIV-positive and whose CD4 level was 200-500 cells/µL (57).
Overall, feeding yeast β-glucan to high-risk patients, such as those who have undergone surgery, can reduce the risk of infection. However, the precise effect is unclear. Several problems, such as controlling the reaction, remain to be solved.
There have been 22 randomized controlled studies on the use of β-glucan in cancer treatment. All were carried out in Japan and most were published in Japanese in 2000 or before.
Among the studies, nine were on stomach cancer, of which six used sizofirnan (SPG) and three used lentinan. Four of the studies on stomach cancer used in patients with inoperable or recurrent cancer (58-61), and the survival time of the subjects was extended in all four cases. Three studies were carried out on operable or postoperative cases (62-64) but no effect regarding survival was observed except in one case of postoperative administration. The remaining two studies did not state the stage of stomach cancer but reported that the survival time of the subjects was extended significantly (65, 66).
There were six randomized controlled studies with uterine cancer patients. Sizofirnan (SPG) was administered in all cases. One study (67) was applied to patients at stage IB-IV, and the others were applied to patients at stages II or III (68-72). They compared the survival time, complete remission rate, recurrence, the size of tumor, etc., with positive results being obtained in most cases.
In three studies, β-glucan was administered to patients with cancers other than stomach or cervical cancer. Among 69 patients with metastatic prostate cancer, those given lentinan together with hormone and anticancer chemotherapy showed significant improvement compared with the control group with respect to the 50% survival time and five-year survival rate (73). Among the 33 patients with recurrent breast cancer, those who were injected with lentinan (LNT), postoperatively, showed slower tumor growth than the control group (74).
Overall, yeast glucan administered to cancer patients can enhance the effect of anticancer chemotherapy or radiation therapy and has positive effects on the survival and quality of life of cancer patients. However, as stated above, most studies were carried out in one country only, Japan, and by similar research groups. Given that subjects from a single country cannot provide an adequate sample and that similar research groups are likely to make the same types of error (if any were made), it would be wise to confirm these results by other researchers in other countries.
β-glucan, which is a polysaccharide in the form of fiber, is the main element of fiber in grains such as oats, barley, yeast and mushrooms. There have been several studies on the efficacy of β-glucan focusing mainly on the lipid lowering effects, blood sugar reduction, weight reduction, immune modulator, and anticarcinogenic effects (Table 2).
Cereal β-glucan and yeast β-glucan were discovered separately but were found to be similar. However, cereal β-glucan is mainly used to reduce the risk of cardiac diseases by lowering the cholesterol level and controlling blood sugar. On the other hand, yeast β-glucan is used to enhance the immune system to fight against cancer and prevent infections.
The results of several clinical experiments show that the increased intake of β-glucan through oat or barley generally reduces the cholesterol level. However, some clinical experiments failed to show such an effect. An attempt was made to explain the inconsistency through the following factors: differences in the β-glucan dose, the molecular size of β-glucan, the composition of food, the process of food preparation and the initial variation in the cholesterol level. However, no single factor can adequately explain the inconsistency. In addition, the results of research into the dose-response and long-term effects are inconsistent.
With regard to the control of blood sugar, there have been many positive results in diabetic patients but they have been mainly negative in nondiabetics. With regard to barley, there has been no randomized controlled study that observed the blood sugar levels in diabetic patients, so it is not possible to draw any conclusions. Moreover, there is a need to explain whether the effect of oats on sugar control arises from its dietary fiber or the dose-response, dosage-dependency, and long-term effects. Some clinical experiments have examined the effect of β-glucan on weight. However, because their primary concern was not weight reduction, they did not provide clear and precise information regarding the effect on weight.
A number of clinical experiments examined whether or not β-glucan in yeast or mushroom prevents an infection or cancer. If yeast β-glucan is given to high-risk patients, such as those who have undergone surgery, the risk of an infection may be reduced. However, it is not known what the effect will be, and the problem of a stable response remains to be solved. Yeast β-glucan it can enhance the effect of anticancer chemotherapy or radiation therapy and have a positive effect on the survival and quality of life of cancer patients. However, as stated above, most of this research was carried out in only one country, Japan, and by similar research groups. Therefore, more study by other scientists in other countries will be needed.
Figures and Tables
Table 1
Summary of Food and Drug Administration labeling on oats and coronary heart disease*

*FDA TALK PAPER. FDA allows whole Oat foods to make health claim on reducing the risk of heart disease, 1997. Available at: http://www. cfsan.fda.gov/~lrd/tpoats.html. [Accessed August 21, 2006].
References
1. Hendler SS, Rorvik D. PDR for Nutritional Supplements. 2001. 1st ed. Thomson Healthcare.
2. Anderson JW, Gilinsky NH, Deakins DA, Smith SF, O'Neal DS, Dillon DW, Oeltgen PR. Lipid responses of hypercholesterolemic men to oat-bran and wheat-bran intake. Am J Clin Nutr. 1991. 54:678–683.
3. Institute of Medicine of the National Academies. Dietary reference intakes. Proposed definition of dietary fiber. 2001. Washington, DC: National Academies Press.
4. Institute of Medicine of the National Academies. Dietary reference intakes. Energy, carbohydrates, fiber, fat, fatty acids, cholesterol,
protein and amino acids. 2002. Washington, DC: National Academies Press.
5. Wan K. Effects of lentinan of peripheral blood mononuclear cell expression of interleukin-2 receptor in patients with chronic hepatitis B in vivo and in vitro. Hunan Yi Ke Da Xue Xue Bao. 1998. 23:90–92.
6. Nakano T, Oka K, Hanba K, Morita S. Intratumoral administration of sizofiran activates Langerhans cell and T cell infiltration in cervical cancer. Clin Immunol Immunopathol. 1996. 79:79–86.
7. Kupfahl C, Geginat G, Hof H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int Immunopharmacol. 2006. 6:686–696.

8. Wakshull E, Brunke-Reese D, Lindermuth J, Fisette L, Nathans RS, Crowley JJ, Tufts JC, Zimmerman J, Mackin W, Adams DS. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology. 1999. 41:89–107.
9. Kournikakis B, Mandeville R, Brousseau P, Ostroff G. Anthrax-protective effects of yeast beta 1,3 glucans. MedGenMed. 2003. 5:1.
10. Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect Biol Med. 2006. 49:159–170.

11. Beer MU, Arrigoni E, Amado R. Effects of oat gum on blood cholesterol levels in healthy young men. Eur J Clin Nutr. 1995. 49:517–522.
12. Bridges SR, Anderson JW, Deakins DA, Dillon DW, Wood CL. Oat bran increases serum acetate of hypercholesterolemic men. Am J Clin Nutr. 1992. 56:455–459.

13. Lia A, Hallmans G, Sandberg AS, Sundberg B, Aman P, Andersson H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am J Clin Nutr. 1995. 62:1245–1251.

14. Lia A, Andersson H, Mekki N, Juhel C, Senft M, Lairon D. Postprandial lipemia in relation to sterol and fat excretion in ileostomy subjects given oat-bran and wheat test meals. Am J Clin Nutr. 1997. 66:357–365.

15. Bourdon I, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, Knuckles B, Schneeman BO. Postprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am J Clin Nutr. 1999. 69:55–63.
16. Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999. 69:30–42.

17. Ripsin CM, Keenan JM, Jacobs DR Jr, Elmer PJ, Welch RR, Van Horn L, Liu K, Turnbull WH, Thye FW, Kestin M. Oat products and lipid lowering. A meta-analysis. JAMA. 1992. 267:3317–3325.

18. Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P, Collins MW. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur J Clin Nutr. 1994. 48:465–474.
19. Lovegrove JA, Clohessy A, Milon H, Williams CM. Modest doses of beta-glucan do not reduce concentrations of potentially atherogenic lipoproteins. Am J Clin Nutr. 2000. 72:49–55.
20. Torronen R, Kansanen L, Uusitupa M, Hanninen O, Myllymaki O, Harkonen H, Malkki Y. Effects of an oat bran concentrate on serum lipids in free-living men with mild to moderate hypercholesterolaemia. Eur J Clin Nutr. 1992. 46:621–627.
21. Beer MU, Arrigoni E, Amado R. Effects of oat gum on blood cholesterol levels in healthy young men. Eur J Clin Nutr. 1995. 49:517–522.
22. Robitaille J, Fontaine-Bisson B, Couture P, Tchernof A, Vohl MC. Effect of an oat bran-rich supplement on the metabolic profile of overweight premenopausal women. Ann Nutr Metab. 2005. 49:141–148.

23. Karmally W, Montez MG, Palmas W, Martinez W, Branstetter A, Ramakrishnan R, Holleran SF, Haffner SM, Ginsberg HN. Cholesterol-lowering benefits of oat-containing cereal in Hispanic americans. J Am Diet Assoc. 2005. 105:967–970.

24. Kerckhoffs DA, Hornstra G, Mensink RP. Cholesterol-lowering effect of beta-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when beta-glucan is incorporated into bread and cookies. Am J Clin Nutr. 2003. 78:221–227.
25. Leadbetter J, Ball MJ, Mann JI. Effects of increasing quantities of oat bran in hypercholesterolemic people. Am J Clin Nutr. 1991. 54:841–845.

26. De Groot AP, Luyken R, Pikaar NA. Cholesterol-lowering effect of rolled oats. Lancet. 1963. 2:303–304.

27. Reyna NY, Cano C, Bermudez VJ, Medina MT, Souki AJ, Ambard M, Nunez M, Ferrer MA, Inglett GE. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am J Ther. 2003. 10:438–443.

28. Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The hypocholesterolemic effects of beta-glucan in oatmeal and oat bran. A dose-controlled study. JAMA. 1991. 265:1833–1839.

29. Uusitupa MI, Ruuskanen E, Makinen E, Laitinen J, Toskala E, Kervinen K, Kesaniemi YA. A controlled study on the effect of beta-glucan-rich oat bran on serum lipids in hypercholesterolemic subjects: relation to apolipoprotein E phenotype. J Am Coll Nutr. 1992. 11:651–659.

30. Uusitupa MI, Miettinen TA, Sarkkinen ES, Ruuskanen E, Kervinen K, Kesaniemi YA. Lathosterol and other non-cholesterol sterols during treatment of hypercholesterolaemia with beta-glucan-rich oat bran. Eur J Clin Nutr. 1997. 51:607–611.

31. McIntosh GH, Whyte J, McArthur R, Nestel PJ. Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr. 1991. 53:1205–1209.

32. Lupton JR, Robinson MC, Morin JL. Cholesterol-lowering effect of barley bran flour and oil. J Am Diet Assoc. 1994. 94:65–70.

33. Behall KM, Scholfield DJ, Hallfrisch J. Lipids significantly reduced by diets containing barley in moderately hypercholesterolemic men. J Am Coll Nutr. 2004. 23:55–62.

34. Keogh GF, Cooper GJ, Mulvey TB, McArdle BH, Coles GD, Monro JA, Poppitt SD. Randomized controlled crossover study of the effect of a highly beta-glucan-enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am J Clin Nutr. 2003. 78:711–718.
35. Nicolosi R, Bell SJ, Bistrian BR, Greenberg I, Forse RA, Blackburn GL. Plasma lipid changes after supplementation with beta-glucan fiber from yeast. Am J Clin Nutr. 1999. 70:208–212.
36. Anderson JW, Randles KM, Kendall CW, Jenkins DJ. Carbohydrate and fiber recommendations for individuals with diabetes: a quantitative assessment and meta-analysis of the evidence. J Am Coll Nutr. 2004. 23:5–17.

37. Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003. 26:2261–2267.

38. American Diabetes Association. Nutrition Principles and Recommendations in Diabetes. Diabetes Care. 2004. 27:Suppl 1. S36–S46.
39. Tapola N, Karvonen H, Niskanen L, Mikola M, Sarkkinen E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2005. 15:255–261.

40. Jenkins AL, Jenkins DJ, Zdravkovic U, Wursch P, Vuksan V. Depression of the glycemic index by high levels of beta-glucan fiber in two functional foods tested in type 2 diabetes. Eur J Clin Nutr. 2002. 56:622–628.
41. Pick ME, Hawrysh ZJ, Gee MI, Toth E, Garg ML, Hardin RT. Oat bran concentrate bread products improve long-term control of diabetes: a pilot study. J Am Diet Assoc. 1996. 96:1254–1261.
43. Li J, Kaneko T, Qin LQ, Wang J, Wang Y. Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition. 2003. 19:926–929.

44. Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkanen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr. 2002. 75:254–262.

45. Lissner L, Lindroos AK, Sjostrom L. Swedish obese subjects (SOS): an obesity intervention study with a nutritional perspective. Eur J Clin Nutr. 1998. 52:316–322.

46. Kimm SY. The role of dietary fiber in the development and treatment of childhood obesity. Pediatrics. 1995. 96:1010–1014.
47. Alfieri M, Pomerleau J, Grace DM, Anderson L. Fiber intake of normal weight, moderately obese and severely obese subjects. Obes Res. 1995. 3:541–547.

48. Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003. 78:920–927.

49. Birketvedt GS, Aaseth J, Florholmen JR, Ryttig K. Long-term effect of fibre supplement and reduced energy intake on body weight and blood lipids in overweight subjects. Acta Medica (Hradec Kralove). 2000. 43:129–132.
50. Hays NP, Starling RD, Liu X, Sullivan DH, Trappe TA, Fluckey JD, Evans WJ. Effects of an ad libitum low-fat, high-carbohydrate diet on body weight, body composition, and fat distribution in older men and women: a randomized controlled trial. Arch Intern Med. 2004. 164:210–217.
51. Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001. 59:129–139.

52. Babineau TJ, Marcello P, Swails W, Kenler A, Bistrian B, Forse RA. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994. 220:601–609.

53. Babineau TJ, Hackford A, Kenler A, Bistrian B, Forse RA, Fairchild PG, Heard S, Keroack M, Caushaj P, Benotti P. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994. 129:1204–1210.

54. Betafectin Gastrointestinal Study Group. Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Arch Surg. 1999. 134:977–983.
55. Hamano K, Gohra H, Katoh T, Fujimura Y, Zempo N, Esato K. The preoperative administration of lentinan ameliorated the impairment of natural killer activity after cardiopulmonary bypass. Int J Immunopharmacol. 1999. 21:531–540.

56. de Felippe Junior J, da Rocha e Silva Junior M, Maciel FM, Soares Ade M, Mendes NF. Infection prevention in patients with severe multiple trauma with the immunomodulator beta 1-3 polyglucose (glucan). Surg Gynecol Obstet. 1993. 177:383–388.
57. Gordon M, Guralnik M, Kaneko Y, Mimura T, Goodgame J, DeMarzo C, Pierce D, Baker M, Lang W. A phase II controlled study of a combination of the immune modulator, lentinan, with didanosine (ddI) in HIV patients with CD4 cells of 200-500/mm3. J Med. 1995. 26:193–207.
58. Nakao I, Uchino H, Orita K, Kaido I, Kimura T, Goto Y, Kondo T, Takino T, Taguchi T, Nakajima T, Fujimoto S, Miyazaki T, Miyoshi A, Yachi A, Yoshida K, Ogawa N, Furue H. Clinical evaluation of schizophyllan (SPG) in advanced gastric cancer-a randomized comparative study by an envelope method. Gan To Kagaku Ryoho. 1983. 10:1146–1159.
59. Furue H, Uchino H, Orita K, Kimura T, Goto Y, Kondo T, Sato S, Takino T, Taguchi T, Nakao I. Clinical evaluation of schizophyllan (SPG) in advanced gastric cancer (the second report): a randomized controlled study. Gan To Kagaku Ryoho. 1985. 12:1272–1277.
60. Fujimoto S. Clinical efficacies of schizophyllan (SPG) on advanced gastric cancer. Nippon Geka Gakkai Zasshi. 1989. 90:1447–1450.
61. Kanagawa Lentinan Research Group. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Hepatogastroenterology. 1999. 46:2662–2668.
62. Fujimoto S, Orita K, Kimura T, Kondo T, Taguchi T, Yoshida K, Ogawa N, Furue H. Clinical evaluation of SPG (schizophyllan) as a therapeutic adjuvant after surgery of gastric cancer: controlled study by an envelope method. Gan To Kagaku Ryoho. 1983. 10:1135–1145.
63. Fujimoto S, Furue H, Kimura T, Kondo T, Orita K, Taguchi T, Yoshida K, Ogawa N. Clinical evaluation of schizophyllan adjuvant immunochemotherapy for patients with resectable gastric cancer: a randomized controlled trial. Jpn J Surg. 1984. 14:286–292.

64. Fujimoto S, Furue H, Kimura T, Kondo T, Orita K, Taguchi T, Yoshida K, Ogawa N. Clinical outcome of postoperative adjuvant immunochemotherapy with sizofiran for patients with resectable gastric cancer: a randomised controlled study. Eur J Cancer. 1991. 27:1114–1118.

65. Taguchi T. Effects of lentinan in advanced or recurrent cases of gastric, colorectal, and breast cancer. Gan To Kagaku Ryoho. 1983. 10:387–393.
66. Wakui A, Kasai M, Konno K, Abe R, Kanamaru R, Takahashi K, Nakai Y, Yoshida Y, Koie H, Masuda H. Randomized study of lentinan on patients with advanced gastric and colorectal cancer. Tohoku Lentinan Study Group. Gan To Kagaku Ryoho. 1986. 13:1050–1059.
67. Miyazaki K, Mizutani H, Katabuchi H, Fukuma K, Fujisaki S, Okamura H. Activated (HLA-DR+) T-lymphocyte subsets in cervical carcinoma and effects of radiotherapy and immunotherapy with sizofiran on cell-mediated immunity and survival. Gynecol Oncol. 1995. 56:412–420.

68. Cooperative Study Group on SPG for Gynecological Cancer. Clinical effect of sizofiran combined with irradiation in cervical cancer patients: a randomized controlled study. Jpn J Clin Oncol. 1992. 22:17–25.
69. Okamura K, Suzuki M, Chihara T, Fujiwara A, Fukuda T, Goto S, Ichinohe K, Jimi S, Kasamatsu T, Kawai N. Clinical evaluation of sizofiran combined with irradiation in patients with cervical cancer. A randomized controlled study; a five-year survival rate. Biotherapy. 1989. 1:103–107.
70. Okamura K, Hamazaki Y, Yajima A. Adjuvant immunotherapy: two randomized controlled studies of patients with cervical cancer. Biomed Pharmacother. 1989. 43:177–181.

71. Okamura K, Suzuki M, Chihara T, Fujiwara A, Fukuda T, Goto S, Ichinohe K, Jimi S, Kasamatsu T, Kawai N. Clinical evaluation of schizophyllan combined with irradiation in patients with cervical cancer. A randomized controlled study. Cancer. 1986. 58:865–872.

72. Inoue M, Tanaka Y, Sugita N, Yamasaki M, Yamanaka T, Minagawa J, Nakamuro K, Tani T, Okudaira Y, Karita T. Improvement of long-term prognosis in patients with ovarian cancers by adjuvant sizofiran immunotherapy: a prospective randomized controlled study. Biotherapy. 1993. 6:13–18.

73. Tari K, Satake I, Nakagomi K, Ozawa K, Oowada F, Higashi Y, Negishi T, Yamada T, Saito H, Yoshida K. Effect of lentinan for advanced prostate carcinoma. Hinyokika Kiyo. 1994. 40:119–123.
74. Kosaka A, Kuzuoka M, Yamafuji K, Imaizumi A, Hattori Y, Yamashita A. Synergistic action of lentinan (LNT) with endocrine therapy of breast cancer in rats and humans. Gan To Kagaku Ryoho. 1987. 14:516–522.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


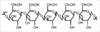

 XML Download
XML Download