Abstract
Radiosurgery has been rarely applied for juvenile nasopharyngeal angiofibroma (JNA) and cumulative reports are lacking. The authors report a case of successful treatment of recurred JNA with gamma knife surgery (GKS). A 48-yr-old man was presented with right visual acuity deterioration and brain magnetic resonance images (MRI) disclosed a 3 cm-sized intraorbital mass in the right orbit. He underwent a right fronto-temporal craniotomy and the mass was subtotally removed to preserve visual function. Histological diagnosis confirmed JNA in typical nature. However, the vision gradually worsened to fail four years after operation. MRI then showed regrowth of the tumor occupying most of the right orbit. GKS was done for the recurred lesion. A dose of 17 Gy was delivered to the 50% isodose line of tumor margin. During the following four-year follow-up period, the mass disappeared almost completely without any complications. Usually JNA can be exclusively diagnosed by radiological study alone. So this report of successful treatment of JNA with GKS may provide an important clue for the novel indication of GKS.
Juvenile nasopharyngeal angiofibroma (JNA) is an uncommon tumor originating primarily in the nasopharynx with extension to surrounding structures such as nasal cavity, sphenoid sinus, sella, pterygomaxillary fossa, infratemporal space, inferior orbital fissure, and intracranial region (1). It represents 0.05% to 0.5% of all head and neck tumors (2,3). Although the tumor is histologically benign, local destructive behaviors of the tumor may lead to serious consequences such as fatal epistaxis and intracranial extension (1). Many modalities have been tried for management of JNA. However, only surgery and external beam radiation therapy were acknowledged as effective treatment modalities for tumor control. Of them, surgery has been the gold standard treatment for JNA up to date (4). Nevertheless, determining optimal modality for advanced lesions continues to be a controversial problem because of complications associated with surgical resection and potential delayed morbidity of radiation therapy (5).
The authors report a successful management of recurred orbital JNA patient with gamma knife surgery (GKS).
A 48-yr-old man was presented with right visual acuity deterioration which had been found in regular medical checkup. Eyesight test revealed 0.4 and 1.2 in right and left eye respectively. There were no other abnormalities in neurological examination including visual field and eye motion. Physical examination revealed mild exophthalmos when observed closely. Brain magnetic resonance images (MRI) disclosed a 3 cm-sized intraorbital mass in the right orbit. He underwent a right fronto-temporal craniotomy with opening of lateral wall and roof of the orbit for direct access to the mass. The mass was subtotally removed to preserve the visual function (Fig. 1). Immediate postoperative visual acuity in right eye was preserved to be 0.5. Histological diagnosis confirmed a diagnosis of JNA in typical nature (Fig. 2). However, the vision gradually became worse and finally failed four years after the operation. Magnetic resonance images then showed regrowth of the tumor occupying most of the right orbit (Fig. 3). As the complete resection of the tumor was a difficult task due to adjacent vital structures, the patient was treated with GKS. Tumor volume was measured 6.8 cm3 and a dose of 17 Gy was delivered to the 50% isodose line of tumor margin (Fig. 4). Because the right vision of the patient was completely lost at the time of GKS, care was taken only to optic chiasm and contra-lateral optic nerve. The maximum radiation dose to this part of optic pathway was 5.4 Gy. Plugs were used to reduce radiation dose to bilateral lenses to decrease the possibility of radiation induced cataract and resultant dose to lenses were 0.8 Gy and 0.4 Gy right and left respectively. The patient tolerated without any complications. Regular follow-up MRI was done annually and revealed marked decrement of the size of tumor successively. The tumor almost completely disappeared four years after GKS (Fig. 5). No complications had developed clinically and radiologically during the follow-up period.
Juvenile nasopharyngeal angiofibroma is a slowly growing tumor that is characterized by submucosal spreading, nonencapsulated, hypervascular, and locally destructive mass (6). Origin of the tumor is superior posterior margin of the sphenopalatine foramen and intracranial invasion appears in 10% to 20% of all patients with JNA and emerge more frequently in the younger adolescent (7). Although JNA exclusively affects adolescent male patients, the diagnosis can be made at any age. There are several reports on giant cell angiofibroma that have middle-age predominance and occur other sites including orbit with histological characteristics of multinucleated giant stromal cells (8,9). However the histology of the authors' case showed that of typical JNA without any features of giant cell angiofibroma.
Treatment of choice of this lesion has been surgery to the present. The aim of surgery is complete resection with minimal morbidity and blood loss. Although the surgical approaches are tailored by tumor stage and location of extension, most of them need aggressive craniofacial exposure. Various methods such as transpalatal, transfacial, transantral, transmaxillary, lateral rhinotomy, infratemporal, or Le Fort approaches have been used for the resection of tumor (6,7). In spite of preoperative embolization, occasional patients require intra and postoperative massive transfusion and are exposed to related complications. Many papers reported on serious complication of life-threatening hemorrhage during surgery (7,10,11). Other complications including optic neurovascular injury, cranial nerve damage, meningitis, motor deficits, also had been reported (12,13). Recently, endoscopic surgery has taken some role in limited cases. However, application of endoscopy is allowed only in small-sized tumor without extensive involvement of the infratemporal fossa and cavernous sinus (14). Impressive experience of using external beam radiation therapy as a primary treatment modality for the management of JNA has been reported by the UCLA group (5). Of 27 patients they reported, tumor was controlled in 23 (85%) and four patients were eventually recurred after two to five years. However, long-term complications of radiation therapy occurred in as many as four patients (15%) and consisted of growth retardation, panhypopituitarism, temporal lobe necrosis, cataract, and radiation keratopathy. Also others reported secondary malignancy of the head and neck as a serious adverse effect of radiation therapy of JNA (4,15). Both surgery and radiation have been reported to be highly effective in the treatment and their cure rates are approximately 80% (4,10). While the serious complications of surgical intervention is immediate, the serious sequelae of radiation therapy follows more protracted course, with a latency reported to be as long as 20 yr (5). So, determining optimal therapeutic management between two modalities has been a source of controversy. Cummings (16) and Harwood et al. (17) analyzed the relative cumulative risks of developing serious complications from both modalities in numerical order, and they concluded that the two modes of treatment carried a comparable cumulative risk (1 in 100).
Management of JNA by radiosurgery has rarely been tried. Dare et al. (18) recently reported two cases of successful GKS application in JNA as a booster treatment for residual tumor after surgical resection. They treated residual tumor after surgery located in infratemporal fossa and cavernous sinus respectively with delivery of 20 Gy to the tumor margin. Thirty six months of follow-up revealed no change in size of one patient and regression of the tumor in the other. Namely, they documented control of postoperative residual tumor with GKS without any complications. Intensity-modulated radiation therapy, a similar form of conformal radiation therapy as radiosurgery, has been tried for the management of extensive and recurrent JNA and is reported to result in favorable outcome (19). Except for the report of Dare et al. (18), no report about radiosurgery on JNA could be found in English literatures. The authors think this is the first case to document complete regression of the pathologically proven JNA after GKS as a primary treatment. In fact, little is known about mechanism of radiation treatment for JNA. However, considering that JNA is composed of the mass with high vascularity that is mainly hyperplastic capillaries, endothelial proliferation by radiation effect may play a key role to result in vascular obliteration and successive regression of the mass.
Radiosurgery has several advantages over surgery or radiation therapy. Radiosurgery is not as invasive as to surgery that is almost free of acute complications in surgery including massive hemorrhage. Besides it is completed in a single-session of a day treatment with no need to prolonged hospital stay. With regard to delayed complications, radiosurgery has less chance of risk than conventional radiation therapy theoretically because of more accurate dosimetric planning is possible with minimal radiation delivery to the normal brain. The safety of radiosurgery in the management of head and neck tumors have confirmed in recent reports (20). McCombe et al. (21) reported that typically, JNA recurs within six to 36 months. So our follow-up period of four years is thought to be not insufficient for determining control of the tumor and emergence of delayed complications by GKS.
Usually JNA can be exclusively diagnosed by radiological study alone. So this report of successful treatment of JNA with GKS may provide another indication of GKS. However, further experiences and studies are required to confirm usefulness of GKS on JNA.
Figures and Tables
Fig. 1
Preoperative axial Gd-DTPA-enhanced T1-weighted magnetic resonance image (A) demonstrating homogeneously enhancing mass in the right orbit. This mass was subtotally removed with small residual remaining in the orbital cone as postoperative computed tomography shows (B).
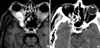
Fig. 2
Histopathological appearance of juvenile nasopharyngeal angiofibroma. Note typical feature of dense fibro-colllagenous tissue with interspersed slit-like and gaping vascular channels (H&E, ×45).

Fig. 3
Axial (A) and sagittal (B) Gd-DTPA-enhanced T1-weighted magnetic resonance images obtained four years after the initial surgery. Multilobulated homogeneously enhancing recurrent mass in the right orbit extending to the inferior orbital fissure and cavernous sinus is shown.
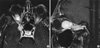
Fig. 4
Axial Gd-DTPA-enhanced T1-weighted magnetic resonance image showing dose distribution for gamma knife surgery. Total volume is measured 6.8 cm3. A dose of 17 Gy was delivered to the 50% isodose line of tumor margin.

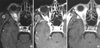
Fig. 5
Serial follow-up Gd-DTPA-enhanced T1-weighted magnetic resonance images after gamma knife surgery. Gradual decrease in size of the mass is noted after a year with total volume of 2.2 cm3 (A) and two years with 0.97 cm3 (B), and finally disappeared four years after the gamma knife surgery (C).
References
1. Neel HB 3rd, Whicker JH, Devine KD, Weiland LH. Juvenile angiofibroma. Review of 120 cases. Am J Surg. 1973. 26:547–556.
2. Gullane PJ, Davidson J, O'Dwyer T, Forte V. Juvenile angiofibroma:a review of the literature and a case series report. Laryngoscope. 1992. 102:928–933.
4. Cummings BJ, Blend R, Keane T, Fitzpatrick P, Beale F, Clark R, Garrett P, Harwood A, Payne D, Rider W. Primary radiation therapy for juvenile nasopharyngeal angiofibroma. Laryngoscope. 1984. 94:1599–1605.

5. Lee JT, Chen P, Safa A, Juillard G, Calcaterra TC. The role of radiation in the treatment of advanced juvenile angiofibroma. Laryngoscope. 2002. 112:1213–1220.

6. Scholtz AW, Appenroth E, Kammen-Jolly K, Scholtz LU, Thumfart WF. Juvenile nasopharyngeal angiofibroma: management and therapy. Laryngoscope. 2001. 111:681–687.

7. Jafek BW, Nahum AM, Butler RM, Ward PH. Surgical treatment of juvenile nasopharyngeal angiofibroma. Laryngoscope. 1973. 83:707–720.

8. Dei Tos AP, Seregard S, Calonje E, Chan JK, Fletcher CD. Giant cell angiofibroma. A distinctive orbital tumor in adults. Am J Surg Pathol. 1995. 19:1286–1293.
9. Guillou L, Gebhard S, Coindre JM. Orbital and extraorbital giant cell angiofibroma: A giant cell-rich variant of solitary fibrous tumor? Clinicopathologic and immunohistochemical analysis of a series in favor of a unifying concept. Am J Surg Pathol. 2000. 24:971–979.
10. Jafek BW, Krekorian EA, Kirsch WM, Wood RP. Juvenile nasopharyngeal angiofibroma: management of intracranial extension. Head Neck Surg. 1979. 2:119–128.

11. Jereb B, Anggard A, Baryd I. Juvenile nasopharyngeal angiofibroma: a clinical study of 69 cases. Acta Radiol Ther Phys Biol. 1970. 9:302–310.

12. Deschler DG, Kaplan MJ, Boles R. Treatment of large juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 1992. 106:278–284.

13. Krekorian EA, Kato RH. Surgical management of nasopharyngeal angiofibroma with intracranial extension. Laryngoscope. 1977. 87:154–164.

14. Nicolai P, Berlucchi M, Tomenzoli D, Cappiello J, Trimarchi M, Maroldi R, Battaglia G, Antonelli AR. Endoscopic surgery for juvenile angiofibroma: When and how. Laryngoscope. 2003. 113:775–782.

15. Park KS, Lee YG, Park HS, Kim JB. Radiation-induced osteosarcoma: report of a case. J Korean Assoc Maxillofac Plast Reconstr Surg. 1998. 20:379–382.
16. Cummings BJ. Relative risk factors in the treatment of juvenile nasopharyngeal angiofibroma. Head Neck Surg. 1980. 3:21–26.

17. Harwood AR, Cummings BJ, Fitzpatrick PJ. Radiotherapy: tumors of the head and neck. J Otolaryngol. 1984. 13:391–394.
18. Dare AO, Gibbons KJ, Proulx GM, Fenstermaker RA. Resection followed by radiosurgery for advanced juvenile nasopharyngeal angiofibroma: report of two cases. Neurosurgery. 2003. 52:1207–1211.

19. Kuppersmith RB, Teh BS, Donovan DT, Mai WY, Chiu JK, Woo SY, Butler EB. The use of intensity modulated radiotherapy for the treatment of extensive and recurrent juvenile angiofibroma. Int J Pediatr Otorhinolaryngol. 2000. 52:261–268.

20. Benk V, Clark BG, Souhami L, Algan O, Bahary J, Podgorsak EB, Freeman CR. Stereotactic radiation in primary brain tumors in children and adolescents. Pediatr Neurosurg. 1999. 31:59–64.

21. McCombe A, Lund VJ, Howard DJ. Recurrence in juvenile angiofibroma. Rhinology. 1990. 28:97–102.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download