Abstract
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome associated with anticonvulsant drugs is a rare but potentially life-threatening disease that occurs in response to arene oxide producing anticonvulsant such as phenytoin and carbamazepine. There have been many reports of cross reactivity among the anticonvulsants upon first exposure to the offending drugs. However, there has been few data describing the development of DRESS syndrome after switching medication from previously well-tolerated phenytoin to carbamazepine, and the induction of hypersensitivity to phenytoin by DRESS to carbamazepine. We experienced a case of a 40-yr-old man who had uncontrolled seizure that led to the change of medication from the long-term used phenytoin to carbamazepine. He developed DRESS syndrome after changing the drugs. We stopped carbamazepine and restored phenytoin for seizure control, but his clinical manifestations progressively worsened and he recovered only when both drugs were discontinued. Patch tests with several anticonvulsants showed positive reactions to both carbamazepine and phenytoin. Our case suggests that hypersensitivity to a previously tolerated anticonvulsant can be induced by DRESS to another anticonvulsant, and that the patch test may be a useful method for detecting cross-reactive drugs in anticonvulsant-associated DRESS syndrome.
Hypersensitivity syndrome is an idiosyncratic, serious drug reaction that consists of a rash, fever, involvement of multiple visceral organs, and hematological abnormalities such as eosinophilia. To better individualize drug hypersensitivity reaction and to distinguish the hypersensitivity reaction from drug-induced pseudolymphoma, Bocquet et al. have recently introduced the term drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (1). Anticonvulsants are the principal drugs responsible for this malady. Among them, arene oxide producing aromatic anticonvulsants such as phenytoin and carbamazepine are the particular drugs most frequently implicated for DRESS syndrome or the anticonvulsant hypersensitivity syndrome (AHS) (2-10).
Cross-reactivity among the aromatic anticonvulsants frequently occurs when a previous history of DRESS syndrome exists (10-12). Many studies have described cross sensitivity as a worsening of the initial features of DRESS syndrome when switching from a sensitive anticonvulsant to a cross-reactive anticonvulsant (8,13-15). However limited data have described the development of DRESS syndrome after switching medication from a previously well-tolerated anticonvulsant to another (5-7).
We present here a case of DRESS syndrome in which hypersensitivity reaction to a previously well-tolerated phenytoin was induced by hypersensitivity to carbamazepine, and we show that the patch test may be a useful method for detecting possible cross-reactive drugs in such situations.
A 40-yr-old man suffering from epilepsia presented with a generalized skin rash, facial edema, sore throat and high-grade fever of 3-day duration. He had been taken phenytoin and sodium valproate for 4 yr without having any adverse reaction. Because of several attacks of seizure while taking these anticonvulsants, the medications were switched to carbamazepine and sodium valproate 16 weeks before the onset of skin rash and fever.
At admission his temperature was 38.9℃, blood pressure 90/50 mmHg, pulse 140 beats/min, and respiratory rate 17 breaths/min. On physical examination, generalized, diffuse, maculopapular, erythematous, pruritic rash was noted over the face, trunk and extremities with marked facial edema (Fig. 1). He had moderately enlarged tonsils, injected oropharynx, and bilateral cervical lymphadenopathy. His neurological examination and the remainder of the physical examination were unremarkable.
The laboratory studies showed a leukocytosis of 16.1×109/L with an eosinophilia of 2.7×109/L (Table 1). The platelet count and coagulation profile were normal. The creactive protein was high at 11.3 mg/dL (normal <0.3 mg/dL), and the erythrocyte sedimentation rate was 2 mm in the first hour. Liver function tests revealed aspartate aminotransferase 68 IU/L (normal 10-59 IU/L), alanine aminotransferase 122 IU/L (normal 10-72 IU/L), alkaline phosphatase 232 IU/L (normal 30-140 IU/L), and serum bilirubin 0.4 mg/dL. Serology for antinuclear factor, RA factor, HBS Ag, anti-HIV antibodies, anti-Mycoplasma antibodies, IgM anti-EB virus antibodies, anti-Hantan virus antibodies, anti-leptospiral antibodies, anti-rickettsial antibodies, p-ANCA and c-ANCA antibodies was all negative. The other laboratory tests were all negative. Skin biopsy showed moderate perivascular and interstitial infiltration of lymphocytes, eosinophils with exocytosis, basal cell vacuolization and marked upper dermal edema.
The diagnosis of DRESS syndrome due to carbamazepine was proposed on the basis of his clinical and biological findings. Carbamazepine was discontinued, phenytoin was restarted at the dose of 300 mg/day, and he was also treated with prednisolone (40 mg/day). However, during the next 7 days his skin rashes and facial edema progressively worsened, and mucosal lesions developed with the persistence of fever (Fig. 2). Rapid progression of the leukocytosis with an eosinophilia was also noted (Table 1). On the seventh day of hospitalization, phenytoin was stopped while continuing prednisolone based on a presumed diagnosis of hypersensitivity reaction to phenytoin. Topiramate (100 mg/day) was then added for seizure control. There was marked improvement in his general condition over the next several days with subsidence of the fever. Following the resolution of his skin eruption and laboratory abnormalities, the prednisolone was tapered over the next 3 weeks.
After obtaining informed consent, patch test was performed 3 months after the total resolution of his illness. Several anticonvulsants including carbamazepine and phenytoin were tested at concentrations of 1% and 10% in petrolatum with/without 1% and 10% in water. Using Finn Chambers on Scanopor tape, 2-day closed patch testing was performed on the backs of the patient. The reactions were scored at 30 min after removal and 4 days after application, according to the International Contact Dermatitis Research group recommendations (16,17). Carbamazepine and phenytoin patch tests were positive at day 2 and day 4, however, phenobarbital, sodium valproate, topiramate, gabapentin, and vigabatrin showed no significant response on the patch tests (Fig. 3, Table 2).
At a follow-up examination after 12 months, he was completely asymptomatic and was tolerating sodium valproate (1,200 mg/day) and topiramate (250 mg/day).
DRESS syndrome can manifest 1-8 weeks after starting the anticonvulsant therapy with a mean of 3 weeks (11-13,18). In previously sensitized individuals, DRESS may occur within 1 day upon rechallenge. In our case, DRESS syndrome developed 16 weeks after the introduction of carbamazepine. The patient had no known exposure to other medications that have been implicated in development of the DRESS syndrome. Various factors may have contributed to this long induction period; interindividual variations, individual susceptibility, and induction of the carbamazepine metabolism via the hepatic cytochrome P-450 system by the previously administered phenytoin, which might have reduced the tendency to develop DRESS syndrome.
Cross sensitivity among the aromatic anticonvulsants occurs at a rate as high as 80% (11,12). However, development of DRESS syndrome to one anticonvulsant after switching medication, and the subsequent development of hypersensitivity to a previously well-tolerated anticonvulsant upon second exposure rarely occurs (5-7). For most of these cases, there was no objective evidence about secondary induction of hypersensitivity to a previously tolerated drug, and only a presumptive diagnosis was made based on the clinical manifestations (5,7). Some patients with DRESS syndrome may have flare-up of symptoms 3 to 4 weeks after the initiation of the reaction despite the discontinuation of the offending drug. In addition, once the offending drug is discontinued, those organs initially involved may show progressive changes or organs that were previously uninvolved may manifest involvement (11). Therefore, we cannot exclude the possibility that the worsening of the clinical features was not due to cross-reactivity but due to the natural course of the DRESS syndrome in some reports (5,7). Klassen and Sadler (6) only showed induction of hypersensitivity to the previously tolerated phenytoin by carbamazepine with the reintroduction of phenytoin to the patient. The initial lack of adverse reaction to phenytoin, followed by immediate adverse response on later rechallenge several months after development of hypersensitivity to carbamazepine indirectly suggest induction of hypersensitivity to phenytoin in that report. Besides the clinical features, we showed induction of hypersensitivity to phenytoin with the results of the patch tests.
The diagnosis of DRESS syndrome is made based on the history of drug exposure and clinical examination. The differential diagnosis includes other cutaneous drug reactions, acute infections, neoplastic, and other immunologic disorders. Withdrawal of the suspicious drug and subsequent improvement of clinical manifestations makes the diagnosis more reliable. When anticonvulsant therapy is invaluable, however, additional diagnostic methods can be sought to select safe drugs for seizure control. Although no gold standard exists, in vitro lymphocyte toxicity assay or lymphocyte transformation tests (LTT), and in vivo patch tests may be helpful in such situations. Many studies have showed the usefulness of LTT and patch testing for the diagnosis of hypersensitivity to anticonvulsants (11,12,17-23). LTT shows similar results with patch test. But false negative reaction of LTT was also noted in patients with simultaneous positive patch test (20). As patch testing is less cumbersome and seems more reliable, it is more frequently used in the case of diagnostic uncertainty (17,19-24). Positive rate of patch tests to carbamazepine were relatively high from 70% to 100% (20-24). However, positive patch test results in phenytoin-induced AHS were much lower (30-60%) than in carbamazepine-induced AHS (25,26). Moreover, it is not yet known accurately how many patients who are on anticonvulsants have a positive patch test. Thus the diagnostic accuracy of patch test in DRESS syndrome is currently unknown. In general, positive predictive value of patch test is relatively good, but negative results of patch test cannot exclude the possibility of hypersensitivity. If patch testing is to be performed, 1% and 10% carbamazepine or phenytoin in petrolatum, in water or in alcohol is recommended (11,19). It is also recommended that at least 2 months should elapse from the time of the skin eruption to the testing date since either false positive reactions due to increased reactivity or false negative reactions due to a refractory state may exist. We performed patch tests 3 months after the total resolution of symptoms with several anticonvulsants at 1% and 10% concentrations, and both carbamazepine and phenytoin showed positive results. Although we did not perform an oral rechallenge testing with phenytoin, the imputability of these two drugs was possible because of the clinical features and the in vivo patch test results.
Aromatic anticonvulsants are metabolized by the cytochrome P-450 enzyme to a common arene oxide metabolite that is normally detoxified by enzyme systems such as epoxide hydrolase. Genetically determined abnormalities in enzyme systems leading to inability to detoxify toxic metabolites may be involved in the pathogenesis of AHS (11,12,25). Reactive toxic metabolites irreversibly modify cellular proteins, and then initiate or serve as targets for an immune attack on modified proteins in target organs (27,28). Thus both pharmacogenetic and immunologic mechanism may play an important role in anticonvulsant-induced DRESS syndrome, and this immune response can explain the late induction of hypersensitivity to the previously tolerated phenytoin after sensitization to carbamazepine. Recent studies have strongly suggested that viral infections, especially reac-tivation of human herpesvirus 6, contribute to the pathogen-esis of drug hypersensitivity to anticonvulsants (4,9,10). However further study will be required to establish the relationship between human herpesvirus 6 infection and drug hypersensitivity.
In conclusion, we present a case of carbamazepine-induced DRESS syndrome that also showed induction of hypersensitivity to the previously well-tolerated phenytoin. Our case suggests that physician should be aware that hypersensitivity to previously tolerated anticonvulsants can be induced by hypersensitivity to another anticonvulsant, and patch test may be a worthwhile method for detecting other possible cross-reactive drugs in such situations.
Figures and Tables
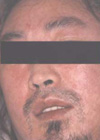 | Fig. 1Generalized diffuse maculopapular rashes over the face and trunk with facial edema on the day of admission. |
 | Fig. 2Desquamative facial rash and edema (A), and vesiculation on upper arms (B). Photographs taken at seventh admission day with prednisolone (40 mg/day) treatment. |
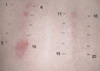 | Fig. 3Results of drug patch testing at day 2. 1, petrolatum; 2, phenytoin 10% aq; 3, phenytoin 1% aq; 4, phenobarbital 10% pet; 5, phenobarbital 1% pet; 6, phenytoin 10% pet; 7, phenytoin 1% pet; 8, carbamazepine 10% aq; 9, carbamazepine 1% aq; 10, carbamazepine 10% pet; 11, carbamazepine 1% pet; 12, carbamazepine 0.5% pet; 13, carbamazepine 0.1% pet; 14, sodium valproate 10% pet; 15, sodium valproate 1% pet; 16, topiramate 10% pet; 17, topiramate 1% pet; 18, gabapentin 10% pet; 19, gabapentin 1% pet; 20, vigabatrin 10% pet. aq, in water; pet, in petrolatum. |
References
1. Bocquet H, Bagot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS). Semin Cutan Med Surg. 1996. 15:250–257.

2. Jee YK, Kim WK, Kim JS, Kim YY, Cho SH, Min KU, Kim YK, Song SH. A case of anticonvulsant hypersensitivity syndrome induced by carbamazepine. Korean J Allergy. 1995. 15:90–95.
3. Park SJ, Kang SB, Lee SH, Jung DY, Yoo JH, Kim JY, Park IW, Choi BW. A case of anticonvulsant hypersensitivity syndrome with pseudolymphoma induced by carbamazepine. J Asthma Allergy Clin Immunol. 2001. 21:657–661. [in Korean].
4. Kim JW, Kim JS, Kim KJ. A clinical observation of drug hypersensitivity syndrome and serologic and molecular genetic analyses of human herpesvirus-6 reactivation. Korean J Dermatol. 2005. 43:143–150.
5. Allam JP, Paus T, Reichel C, Bieber T, Novak N. DRESS syndrome associated with carbamazepine and phenytoin. Eur J Dermatol. 2004. 14:339–342.
6. Klassen BD, Sadler RM. Induction of hypersensitivity to a previously tolerated antiepileptic drug by a second antiepileptic drug. Epilepsia. 2001. 42:433–435.
7. Kaur S, Sarkar R, Thami GP, Kanwar AJ. Anticonvulsant hypersensitivity syndrome. Pediatr Dermatol. 2002. 19:142–145.

8. Galindo PA, Borja J, Gomez E, Mur P, Gudin M, Garcia R, Encinas C, Romero G, Garrido JA, Cortina P, Feo F. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol. 2002. 12:299–304.
9. Conilleau V, Dompmartin A, Verneuil L, Michel M, Leroy D. Hypersensitivity syndrome due to 2 anticonvulsant drugs. Contact Dermatitis. 1999. 41:141–144.

10. Descamps V, Bouscarat F, Laglenne S, Aslangul E, Veber B, Des-camps D, Saraux JL, Grange MJ, Grossin M, Navratil E, Crickx B, Belaich S. Human herpesvirus 6 infection associated with anticon-vulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br J Dermatol. 1997. 137:605–608.

11. Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999. 21:489–501.
12. Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome, in vitro assessment of risk. J Clin Invest. 1988. 82:1826–1832.

13. Vittorio CC, Muglia JJ. Anticonvulsant hypersensitivity syndrome. Arch Intern Med. 1995. 155:2285–2290.

14. Kleier RS, Breneman DL, Boiko S. Generalized pustulation as a manifestation of the anticonvulsant hypersensitivity syndrome. Arch Dermatol. 1991. 127:1361–1364.

15. Nashed MH, Liao L. Possible atypical cross-sensitivity between phenytoin and carbamazepine in the anticonvulsant hypersensitivity syndrome. Pharmacotherapy. 2001. 21:502–505.

16. Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, Hjorth N, Maibach HJ, Malalten KE, Meneghini CL, Pirila V. Terminology of contact dermatitis. Acta Derm Venereol. 1970. 50:287–292.
17. Devos SA, Van Der Valk PG. Epicutaneous patch testing. Eur J Dermatol. 2002. 12:506–513.
18. Pelekanos J, Camfield P, Camfield C, Gordon K. Allergic rash due to antiepileptic drugs: clinical features and management. Epilepsia. 1991. 32:554–559.

19. Barbaud A, Goncalo M, Bruynzeel D, Bircher A. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis. 2001. 45:321–328.
20. Houwerzijl J, De Gast GC, Nater JP, Esselink MT, Nieweg HO. Lymphocyte-stimulation tests and patch tests to carbamazepine hypersensitivity. Clin Exp Immunol. 1977. 29:272–277.
21. Silva R, Machado A, Brandao M, Goncalo S. Patch test diagnosis in carbamazepine erythroderma. Contact Dermatitis. 1986. 15:254–255.

22. Alanko K. Patch testing in cutaneous reactions caused by carbamazepine. Contact Dermatitis. 1993. 29:254–257.

23. Puig L, Nadal C, Fernandez-Figueras MT, Alomar A. Carbamazepine-induced drug rashes: diagnostic value of patch tests depends on clinicopathologic presentation. Contact Dermatitis. 1996. 34:435–437.

24. Jones M, Fernandez-Herrera J, Dorado JM, Sols M, Ruiz M, Garcia-Diez A. Epicutaneous test in carbamazepine cutaneous reactions. Dermatology. 1994. 188:18–20.

25. Lee AY, Kim MJ, Chey WY, Choi J, Kim BG. Genetic polymorphism of cytochrome P450 2C9 in diphenylhydantoin-induced cutaneous adverse drug reactions. Eur J Clin Pharmacol. 2004. 60:155–159.
26. Galindo PA, Borja J, Gomez E, Mur P, Gudin M, Garcia R, Encinas C, Romero G, Garrido JA, Cortina P, Feo F. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol. 2002. 12:299–304.
27. Leeder JS, Riley RJ, Cook VA, Spielberg SP. Human anti-cytochrome P450 antibodies in aromatic anticonvulsant-induced hypersensitivity reactions. J Pharmacol Exp Ther. 1992. 263:360–367.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download