Abstract
We report here on the multiple genital tract neoplasms in a 41-yr-old Korean woman with Peutz-Jeghers Syndrome (PJS). The patient presented with lower abdominal pain. Her previous medical history was PJS and breast cancer. Pelvic ultrasound showed a multilocular cyst at the right adnexal region, diagnosed as bilateral ovarian mucinous borderline tumors. An ovarian sex cord tumor with annular tubules was incidentally diagnosed together with a minimal deviation adenocarcinoma of the uterine cervix and mucinous metaplasia of both the Fallopian tubal mucosa and the endometrium. Although the cases of multiple genital tract tumors with PJS has rarely been reported, the present case appears to be the first in Korea in which the PJS syndrome was complicated by multiple genital tract tumors and infiltrating carcinoma of the breast. The clinical significance of the multiple genital tract tumors and breast cancer associated with PJS is reviewed.
Peutz-Jeghers syndrome (PJS) is an autosomal dominantinherited disorder that is characterized by hamartomatous polyps of the gastrointestinal tract, mucocutaneous melanin pigmentation and various neoplasms. Previous studies have reported that PJS is often accompanied with a higher incidence of other malignancies, such as breast cancer, adenoma malignum of the uterine cervix and ovarian tumors, and the incidence of cancer among the patients suffering with PJS has been estimated to be 15-fold higher than the incidence of cancer in the general population (1-8).
Genital tract neoplasms in the female patients with PJS include ovarian neoplasms from the epithelium and stromal cells, adenoma malignum of the cervix and adenocarcinoma of the endometrium (1). The most common ovarian neoplasm found in patients with PJS is the sex cord tumor with annular tubules (SCTAT), and this is followed by Sertoli cell tumor of the ovary, mucinous epithelial ovarian tumor, serous tumor and ovarian mature teratoma. Each of the genital tract neoplasms in female patients rarely occur in this syndrome, whereas cases with multiple tumors at different sites are very rare indeed.
We report here on a 41-yr-old woman with PJS who had a sex cord tumor with annular tubules, ovarian mucinous borderline tumor, adenoma malignum of the cervix, mucinous metaplasia of both the Fallopian tubal mucosa and the endometrium, and infiltrating carcinoma of the breast.
In 1999, a 41-yr-old, G2P1, Korean woman was referred to our institution because of a right adnexal palpable mass that was associated with lower abdominal pain. In 1989, the patient was diagnosed as having PJS because of the multiple hamartomatous polyps found in the small bowel and the melanin pigmentation around lips. In 1990, a small bowel resection was performed for intussusception. After 1990, findings on endoscopic examinations had been negative up to death.
In 1997, the left modified radical mastectomy and left axillary lymph nodes dissection were performed for breast cancer. The tumor pathological stage was pT2, N1, M0. Postoperatively, the patient had two courses of combination chemotherapy that consisted of 5-fluorouracil, doxorubicin and cyclophosphamide. During chemotherapy, the patient was not able to tolerate the toxicity of chemotherapy and so it was stopped. There was no family history of the PJS. Physical examination revealed freckling around the lips. Pelvic examination revealed an anteverted, slightly enlarged uterus and a right adnexal mass 12 cm in diameter. The Pap smear contained several atypical metaplastic cells. Cervical biopsy was performed and the histological diagnosis revealed adenoma malignum. Pelvic ultrasound showed multiple myomas in the uterus and a multilocular cyst at the right adnexal region that was 15.1×13.8×12.6 cm in size. At the laparotomy, the right and left adnexal cyst appeared to be 20 cm and 8 cm at the maximal diameter, respectively. A subsequent bilateral salpingo-oophorectomy was performed and the intra-operative frozen sections were found to contain mucinous tumors from the both ovaries. Consequently, the patient underwent radical hysterectomy and biopsy of the whitish tissue of the previous colectomy site that was 1 cm in diameter.
Postoperatively, the patient had one course of combination chemotherapy that consisted of cisplatin and 5-fluorouracil. The patient was not able to tolerate the toxicity of chemotherapy and so we stopped it. In 2001, the patient presented with dyspnea. Chest CT scan revealed pleural effusion, extensive consolidations and small metastatic lung nodules in both lungs. Treatment with combination chemotherapy that consisted of of taxol and epirubicin was unsuccessful, and patient expired approximately 4 yr after the radical hysterectomy.
Gastrointestinal tract (1988 to 1990): 21 polyps were biopsied and removed by polypectomy from the colon and stomach once in 1988, three times in 1989 and once in 1990. In 1990, the segmental resection of the strangulated small intestine was done. There were three polyps with the largest one measuring 5.6×3.3×3.0 cm and they showed hemorrhagic infarct (Fig. 1). On the histologic examination, the polyps were hamartomatous polyps.
Breast (1997): The left breast and the axillary fat pad were removed by a modified radical mastectomy after excisional biopsy for frozen sectioning. The breast measured 20.0×12.5×1.5 cm. The nipple was hemorrhagic and eroded. The ill defined, grayish and gritty mass measuring 3.2×2.0×1.0 cm was found on the lower outer quadrant. Histologically, the infiltrating ductal carcinoma was Broom and Richardson grade II, and it involved the nipple and one of 21 regional lymph nodes (Fig. 2).
Female genital tract (1999): Radical hysterectomy and bilateral salpingo-oophorectomy were performed. The uterus measured 12.0×6.0×4.0 cm and it weighed 220 g. The uterine endocervix showed a mucinous solid mass that measured 4.5×2.0 cm (Fig. 3A). Both the ovaries were cystic. The right ovary measured 19.0×13.0×7.0 cm and it weighed 800 g, and the left ovary measured 9.0×6.0×5.0 cm and it weighed 180 g (Fig. 3B). The cut surface showed multilocular cysts containing mucus. Both oviducts were unremarkable on gross examination. The peritoneum, including the hamartomatous polyps around the anastomosis site for the previous segmental resection of small intestine, was removed. Histologically, the uterine cervix showed a deeply infiltrative, but well differentiated mucinous adenocarcinoma (adenoma malignum) (Fig. 4). Frequent sites of vascular invasion were found. The pelvic lymph nodes on both sides showed metastasis. The peritoneum around the anastomosis site showed a pseudomyxoma peritonei. Both the ovaries showed mucinous tumor that had borderline malignancy (Fig. 5A). Additionally, there were scattered small nests of sex cord tumors with annular tubules in the cortex and septa of the multilocular cysts (Fig. 5B). Both the tubal mucosa and endometrium showed mucinous metaplasia (Fig. 6). There was a metastatic adenocarcinoma from the uterine cervix in the pelvic lymph nodes.
The most common ovarian neoplasm found in the patients with PJS is the SCTAT (1). Among the patients diagnosed with SCTAT, 36% of them are affected by PJS (2). The SCTAT is a distinctive ovarian neoplasm, and the predominant component of this tumor has morphologic features that are intermediate between those features of the granulose cell tumor and those features of the Sertoli cell tumor; focal differentiation into either granulose cell or Sertoli cell tumor may occur. SCTAT is capable of producing both estrogen and progesterone (2,3). Symptoms of hyperestrogenism, including irregular menstrual bleeding, postmenopausal bleeding and isosexual precocity, have been described (3). When this condition occurs in association with PJS, the ovarian SCTATs are almost always benign and they are typically multifocal, calcified, bilateral, very small or even microscopical in size (2). Microscopically, ovarian SCTATs with PJS are mainly characterized by sharply circumscribed rounded epithelial nests composed of ring-shaped tubules encircling the hyalinized basement membrane-like material. The nests have two basic patterns: the simple pattern is that of a single tubule encircling a central rounded hyaline mass, and the complex pattern is characterized by communicating tubules encircling multiple hyaline masses. In contrast, those SCTATs unassociated with PJS are unilateral and large, and they sometimes have malignant behavior. Microscopically, the predominant appearance of the tumor is similar to that encountered in patients with the PJS, but variations from the characteristic pattern are seen in minor portions of the tumor.
Another frequent ovarian neoplasm associated with PJS is the Sertoli cell tumor of the ovary that causes precocious puberty (1). Mucinous epithelial ovarian tumors are also seen with increased frequency in patients with PJS. Although the frequency of mucinous ovarian tumors in PJS patients may be close to that seen in the general population, it appears that the ratio of mucinous to serous tumors is higher in PJS patients than the ratio of mucinous to serous tumors in the general population (8:1-1:3, respectively). Two cases of serous tumor of the ovary associated with PJS have been published (3,4). In general, the patients with PJS who have even simple ovarian cysts should undergo frequent follow-up and, at least, annual sonographic surveillance.
Minimal deviation adenocarcinoma (adenoma malignum) of the uterine cervix is a well-differentiated variant of adenocarcinoma in which the branching glandular pattern strongly mimics that of the normal endocervical glands. Adenoma malignum is strongly associated with PJS (1,3,5,6). A serine threonine kinase gene, STK11, has been identified as the tumor suppressor gene responsible for the PJS (9). Kuragaki et al. (9) have reported that somatic mutations of the STK 11 gene were confirmed in 6 (55%) of the 11 mucinous adenoma malignums and in 1 (5%) of the 19 mucinous adenocarcinomas, but not in 5 nonmucinous adenocarcinomas, 15 squamous cell carcinomas, nor in 5 endocervical glands with gastric metaplasia. Adenoma malignums with the STK 11 mutation has a significantly poorer prognosis than adenoma malignums without the STK 11 mutation. Mutations in the STK 11 gene may play an important role in the etiology of adenoma malignum of the uterine cervix, and this gene mutation may distinguish this rare tumor from the other more common types of adenocarcinoma of the uterine cervix.
The prognosis of patients with cervical adenoma malignum associated with PJS is generally poor (3,10). Srivastsa et al. (3) reviewed the outcome of the 10 reported cases of cervical adenoma malignum associated with PJS that had adequate follow-up. Eight of these 10 patients died of their disease, and only one patient survived for more than 5 yr. Of the 26 cases reported by Gilks et al. (10), 13 of the 22 patients (59%) died of their disease and four additional patients were alive with recurrent tumor. They also reviewed a total of 57 cases that had been reported since 1963 and that had at least 2 yr of follow-up. Only 16 patients were alive without recurrent disease; 33 patients died of their disease.
Mucinous metaplasia and primary mucinous neoplasia of the Fallopian tube are extremely rare. Mucinous metaplasia of the Fallopian tube has been associated with PJS (5,11). Seidman (11) in 1994 reported on seven women with mucinous lesions of the Fallopian tube, and two of the patients had PJS. The mucinous lesions of the Fallopian tube were associated with multiple independent mucinous neoplasms and with PJS. Furthermore, they suggested the possibility that mucinous metaplasia of the Fallopian tube was due to the direct extension from a adenoma malignum of the endocervix.
Breast cancer that is usually ductal, but occasionally it is lobular carcinoma, seems to be found in patients with PJS with an increased frequency (4,7). This syndrome is caused by truncating germline mutations in the LKB1 gene (also called STK11 gene) (7). Low LKB1 protein expression in breast cancer correlates with a higher histological grade, a larger tumor size, a progesterone receptor status and the presence of lymph node metastasis (12). Furthermore, low LKB1 expression has been associated with a higher relapse rate and a worse prognosis. LKB1 expression may be a useful prognostic marker for human breast cancer.
The case of PJS with multiple genital tract tumors at different sites was first reported in 1986, and 3 cases of multiple genital tract tumors with PJS have been reported to date (5,6,13). Chen (5) in 1986 reported the first case with multiple genital tract tumors including bilateral ovarian SCTAT, adenoma malignum of the cervix and bilateral ovarian mucinous tumors in a 33-yr-old woman with PJS. Podezaski et al. (6) in 1991 reported a case of SCTAT of the right ovary, mucinous cystadnoma of the left ovary and recurrent adnoma malignum of the cervix in a woman with PJS. Mangili et al. (13) in 2004 reported a case of ovarian mixed serous and mucinous borderline tumor, ovarian microscopic SCTAT, adenoma malignum of the cervix and areas of mucinous metaplasia of the tubal mucosa in a 41-yr-old woman with PJS.
The case with the multiple genital tract tumors and infiltrating breast cancer has not been reported to date. Our patient was the first case in which the PJS syndrome was complicated by ovarian mucinous borderline tumor, ovarian SCTAT, adenoma malignum of the cervix, the mucinous metaplasia of both the Fallopian tubal mucosa and the endometrium, and infiltrating ductal carcinoma of the breast. The cancer risk for those patients affected with PJS is known to be higher than the cancer risk of the general population. The prognosis for the patient with genital tract tumors associated with PJS is poor and so early detection and regular surveillance of the high-risk patients with PJS is crucial.
Figures and Tables
Fig. 1
The Peutz-Jegher polyp composed of hyperplastic, dilated mucous glands and central radiating smooth muscle bundles. Surface epithelium undergoes hemorrhagic necrosis due to intussception (H-E, ×40).

Fig. 3
(A) The uterine endocervix shows a nodular pale pink tumor, infiltrating into deep cervical wall. (B) Bilateral ovarian multilocular cysts contain mucus.
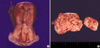
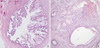
Fig. 4
The endocervical adenocarcinoma composed of well-differentiated, infiltrative glands (H-E, ×100).

References
1. Papageorgiou T, Stratakis CA. Ovarian tumors associated with multiple endocrine neoplasias and related syndromes (Carney complex, Peutz-Jeghers Syndrome, von Hippel Lindau disease, Cowden's disease). Int J Gynecol Cancer. 2002. 12:337–347.
2. Young RH, Welch WR, Dickersin GR, Scully RE. Ovarian sex cord tumor with annular tubules. Review of 74 cases including 27 with Peutz-Jeghers syndrome and four with adenoma malignum of the cervix. Cancer. 1982. 50:1384–1402.

3. Srivatsa PJ, Keeney GL, Podratz KC. Disseminated cervical adenoma malignum and bilateral ovarian sex cord tumors with annular tubules associated with Peutz-Jeghers Syndrome. Gynecol Oncol. 1994. 53:256–264.

4. Dozois RR, Judd ES, Dahlin DC, Bartholomew LG. The Peutz-Jeghers syndrome. Is there a predisposition to the development of intestinal malignancy? Arch Surg. 1969. 98:509–517.
6. Podezaski E, Kaminski PF, Pees RC, Singapuri K, Sorosky JI. Peutz-Jeghers Syndrome with ovarian sex cord tumor with annular tubules and cervical adenoma malignum. Gynecol Oncol. 1991. 42:74–78.
7. de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WT, Oosterwijk JC, Kleibeuker JH, Schaapveld M, de Vries EG. Genes other than BRCA1 and BRCA2 involved in breast cancer susceptibility. J Med Genet. 2002. 39:225–242.

8. Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers Syndrome. Gastroenterology. 2000. 119:1447–1453.

9. Kuragaki C, Enomoto T, Ueno Y, Sun H, Fujita M, Nakashima R, Ueda Y, Wada H, Murata Y, Toki T, Konishi I, Fujii S. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest. 2003. 83:35–45.

10. Gilks CB, Young RH, Aguirre P, DeLellis RA, Scully RE. Adenoma malignum (minimal deviation adenocarcinoma) of the uterine cervix. A clinicopathological and immunohistochemical analysis of 26 cases. Am J Surg Pathol. 1989. 13:717–729.
11. Seidman JD. Mucinous lesions of the fallopian tube [A report of seven cases]. Am J Surg Pathol. 1994. 18:1205–1212.
12. Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002. 8:2085–2090.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download