Abstract
Although germline mutations of met proto-oncogene on human chromosome 7q31-34 have been known as useful molecular markers of hereditary papillary renal cell carcinoma (RCC), the expression of MET, a product of met proto-oncogene, has not been fully studied in sporadic RCC, along with its clinical significance. We investigated the expression of MET by immunohistochemistry in 182 cases of renal neoplasm encompassing 145 RCC, 25 urothelial carcinomas of renal pelvis, and 12 oncocytomas. MET was diffusely and strongly expressed in 90% of papillary RCC, all collecting duct carcinomas, and 92% of urothelial carcinomas of renal pelvis. On the contrary, clear cell RCC, chromophobe RCC, and oncocytomas were negative or focally positive for MET expression. In clear cell RCC, MET expression was positively correlated with high nuclear grade, presence of infiltrative growth, tumoral necrosis, papillary architecture, sarcomatoid component, tumoral involvement of the renal pelvis or ureter, involvement of the calyx, and lymphatic invasion. In conclusion, diffuse and strong expression of MET in papillary RCC and collecting duct carcinoma might be helpful in discriminating from the other subtypes of RCC with tubular or papillary growth. In case of MET expression observed in clear cell RCC, it might correlate with those clinicopathological parameters implying aggressive behavior.
The MET, which is encoded by the met proto-oncogene, is a transmembrane glycoprotein of 190 kDa (1). The ligand of MET is a hepatocyte growth factor/scatter factor (HGF/SF), which has diverse biologic functions including mitogenic (2), motogenic (3) and morphogenetic (4) activities on a variety of epithelial cells, including renal tubular cells (5,6). HGF/SF has been also reported to promote the invasive properties of cultured renal cell carcinoma (RCC) cells in vitro (7), suggesting a possible role of MET in the progression of RCC. The evidences implicating the mutation of met in the development of hereditary papillary renal carcinoma have been obtained (8-10). A gene associated with the hereditary papillary renal carcinoma and a small proportion of sporadic papillary RCC was mapped to a region encompassing the met locus (8), and the sequencing revealed germline met mutations in affected individuals (9). Recently, overexpression of MET protein has been noted in sporadic papillary RCC (11), in which the mutation of met has been known to be rarely detected. The expression of MET in sporadic RCC related to the subtypes, however, has not been well elucidated.
Here, we evaluated the expression of MET by the immunohistochemistry in RCC with various subtypes, urothelial carcinoma of renal pelvis, and renal oncocytoma. We also analyzed the correlation of MET expression with clinicopathologic parameters in clear cell RCC, the most common type of RCC.
Cases obtained by radical nephrectomy due to renal neoplasm were identified from the files of the Department of Pathology, reviewing surgical pathology reports and reexamining all available glass slides of the cases. The RCC classification system proposed by World Health Organization Classification of Tumours in 2004 was adopted (12). Cases included clear cell RCC (n=96), papillary RCC (n=20) including type 1 (n=13) and type 2 (n=7), divided according to the Delahunt and Eble classification (13), chromophobe renal carcinoma (n=24) including typical (n=18) and eosinophilic variant (n= 6), divided according to Thoenes et al. (14), collecting duct carcinoma (n=5), urothelial carcinoma of renal pelvis (n=25), and renal oncocytoma (n=12). Clinical data of the patients with RCC were retrieved. Medical records were reviewed for demographic and follow-up informations. Any cases were excluded if there was family history of RCC or multiple tumors. Macroscopic information pertaining to the tumor size, extent of the tumor, and cystic change was obtained from the pathology reports. The tumor stage at presentation was based on all available clinicopathologic informations according to the scheme proposed by the American Joint Committee on Cancer (15).
Hematoxylin and eosin-stained histologic slides from all cases were reviewed by two pathologists in a blinded independent fashion. A nuclear grade was assigned to each tumor using the grading system of Fuhrman et al. (16).
Immunohistochemical staining was performed on one or two representative blocks per each case with rabbit polyclonal antibody against human MET protein (1:80, Novocastra, Newcastle, U.K.) using the two-step, peroxidase-labeled EnVision+TM system (DAKO, Glostrup, Denmark) after antigen retrieval using two times of heating for 5 min in an 800W microwave oven to maintain the temperature of the buffer (0.01 M citrate, pH 6.0) at about 100℃. Diaminobenzidine was used as a chromogen. Positive internal controls and negative controls (omission of primary antibody) were evaluated simultaneously. It was considered positive if the intercellular or basolateral membranes with or without cytoplasm were stained in more than 5% of tumor cells. And it was graded as 1+ when less than 50% of tumor cells were stained, and as 2+ when 50% or more of tumor cells were stained. The intensity of the staining was graded as weak, moderate, and strong. There were no cases demonstrating less than 50% of tumor cells stained with strong intensity or demonstrating more than 50% of tumor cells stained with weak intensity.
Statistical analysis was performed by use of the Fisher's exact test, Student t-test and analysis of variances at the 5% level in SPSS 10.0 software (SPSS Inc., Chicago, Illinois, U.S.A.).
In normal kidney, MET expression was limited to the epithelial cells in the proximal convoluted tubule, the collecting duct, and the loop of Henle. The glomeruli, distal convoluted tubule, and the stroma were consistently negative for MET expression.
Strong and diffuse MET expression (2+), characteristically localized in the intercellular membranes or basolateral portions with or without cytoplasm of tumor cells, was observed in 90% of papillary RCC, all cases of collecting duct carcinoma, and 92% of urothelial carcinoma (Table 1, Fig. 1A-1C). On the contrary, in clear cell RCC and chromophobe RCC, there was no case with diffuse strong MET expression. There was no expression of MET in 55% of clear cell RCC (Fig. 1D) and 92% of chromophobe RCC. Just focal MET expression (1+) with similar localization was found in 45% of clear cell RCC and 8% of chromophobe RCC (Fig. 2A), but no cases of oncocytoma. There was only faint cytoplasmic staining without membranous accentuation observed in less than 5% of tumor cells in 11 cases of oncocytoma (Fig. 2B) and 11 cases of chromophobe RCC, even after the endogenous biotin blocking.
Focal MET expression (1+) was found in 43 out of 96 clear cell RCC. MET expression in clear cell RCC was correlated with high nuclear grade (p<0.001), presence of infiltrative growth (p<0.001), tumoral necrosis (p<0.001), papillary architecture (p=0.005), sarcomatoid component (p<0.030), tumoral involvement of the renal pelvis or ureter (p=0.014), involvement of the calyx (p=0.016), and lymphatic invasion (p=0.016) (Table 2). MET expression was more frequently found in the tumors of advanced stage (stage 3 or 4) than in localized stage (stage 1 or 2), although it was not statistically significant.
The relationship between MET expression and subtypes of RCC has not been well elucidated in sporadic RCC. In present study, diffuse and strong MET expression was found in papillary RCC and collecting duct carcinoma, whereas clear cell RCC and chromophobe RCC showed negative or focal cytoplasmic staining. Inoue et al. (17) reported that all 20 RCC with tubulo-papillary histology showed positive staining for MET, and 98% (88 out of 90) of clear cell RCC showed negative or focal staining, which well corresponds to our result. There was a few reports showing no association with the subtypes of RCC and MET expression (18,19). The number of cases in those reports, however, is thought to be relatively small to reveal the relationship of the subtypes of RCC and MET expression. Diffuse and strong immunoexpression of MET in papillary RCC and collecting duct carcinoma might be helpful in discriminating from the other subtypes of RCC with tubulo-papillary growth.
Renal neoplasm has been a major subject of cytogenetic and molecular genetic studies. Papillary RCC was characterized by trisomy of chromosomes 7, 16, 17 and loss of Y chromosome in male patients in cytogenetics (20). A germline mutation of met gene of 7q31 has been detected in patients with hereditary papillary renal carcinoma (8) as well as a small portion of sporadic papillary RCC (9). In present study, the over expression of MET was detected in 90% of sporadic papillary RCC by the method of immunohistochemistry, which was much higher than the result of the previous study using molecular method that identified met mutations in 13% of sporadic RCC (9). Our result is consistent with that of a recent study reporting MET over expression in 80% of sporadic RCC by the immunohistochemistry (11). Trisomy of chromosome 7 has been reported in 95% of sporadic RCC (20), and it may result in an increased met mRNA copy number, as described by Glukhova et al. (21). The increased met mRNA copy number may lead to MET overexpression in protein level. The significance of chromosome 7 trisomy observed in sporadic papillary RCC is that the trisomy may serve to increase the dosage of the met allele. The tumorigenesis by met is known to be quantitatively related to its level of activation (22). Thus, it appears that in addition to met mutation, MET overexpression contributes to the sporadic RCC.
Regarding the subtypes of papillary RCC, no significant difference of MET expression between two subtypes was observed in our study. In type 1 and type 2 papillary RCC, 92% and 86% of the cases, respectively, showed immunoexpression of MET (Table 1). Delahunt and Eble proposed subdividing papillary RCC into two subtypes, type 1 with small cells and pale cytoplasm and type 2 with large cells and eosinophilic cytoplasm (13). In the study by Lubensky et al. (23), all 6 sporadic RCC with met mutations demonstrated type 1, whereas 10 papillary RCC without met mutations demonstrated both types equally. In the recent immunohistochemical study by Sweeney et al. (11), however, no significant difference of MET expression between two subtypes was found. In their study, MET was expressed in 81.1% and 76.9% of type 1 and type 2 papillary RCC, respectively, which was consistent with our results. There might be some discrepancies between the changes in molecular level and the immunoexpression of MET in ours.
It is of interest that the diffuse and strong expression of MET was observed in all cases of collecting duct carcinoma, while the majority of chromophobe RCC and all oncocytomas did lack in the expression of MET. The molecular characteristics of renal tumors originating from non-proximal tubules, including collecting duct carcinoma, chromophobe RCC and oncocytoma are not well known. Polascik et al. (24) showed that the loss of heterozygosity of chromosomal arm 3p that is known to be associated with clear cell RCC was infrequent in both collecting duct carcinoma and oncocytoma, and concluded that the molecular events contributing to the development of non-proximal nephron tumors were distinct from those associated with renal tumors originating from the proximal tubules. It is unclear why MET expression was different between in collecting duct carcinoma and in chromophobe RCC or oncocytoma. We suspected that this might be due to the difference in the origin of collecting duct carcinoma and the others. The collecting duct carcinoma is thought to arise from the distal segment of the collecting duct of Bellini, while renal oncocytoma and chromophobe renal carcinoma appears to arise in the cortex from the intercalated cells of the proximal connecting segment of the collecting ducts (25,26). It was not apparent, however, whether the collecting duct of MET overexpression was in the distal segment of Bellini or the proximal connecting segment of the collecting ducts. Further investigations on the staining localization in normal renal structure and molecular studies seem to explain the difference of MET overexpression between collecting duct carcinoma and chromophobe RCC and oncocytoma.
Most cases (92%) of urothelial carcinoma included in our study exhibited strong (2+) MET expression. Li et al. showed MET immunoreactivity in all 49 urothelial carcinomas of the urinary bladder (27) and Tamatani et al. demonstrated that HGF stimulated migration and invasion of the rat tumorigenic urothelial cell lines in vitro (28), while in a study by Natali et al., all 5 urothelial carcinoma of renal pelvis did not display MET expression (19). As pointed out by Pisters et al. (18), this difference may be related to the fact that we used a polyclonal antibody which could theoretically detect more epitopes of the MET protein, whereas the antibody used in the study by Natali et al. (19) was monoclonal antibody against the extracellular domain of the MET protein.
We demonstrated that MET expression in clear cell RCC were correlated with several clinicopathological parameters, such as infiltrative growth pattern, tumoral necrosis, pelvic or ureteral involvement, calyceal involvement, and lymphatic invasion, which were thought to be related to the invasiveness or aggressiveness of the tumor (Table 2). In addition, there was a significant relationship between MET immunoreactivity and the higher Fuhrman's nuclear grade, which is an important prognostic factor (p<0.001). These results are comparable to those of a few studies demonstrating strong expression of MET in more than 80% of RCC with higher grades (18,19). HGF/SF-MET pathway was reported to enhance the invasive properties in the chemoinvasion assay and inhibit Fas-induced apoptosis in vitro (7). In present study, MET expression was more frequently found in the tumors of advanced stage (stage 3 or 4) than in localized stage (stage 1 or 2), and all three clear cell RCC with distant metastasis expressed MET, although it was not statistically significant.
In conclusion, we demonstrated that MET was diffusely and strongly expressed in papillary RCC, collecting duct carcinoma, and urothelial carcinoma of renal pelvis, whereas no or focal expression of MET was observed in clear cell RCC, chromophobe RCC and oncocytoma. Diffuse and strong immunoexpression of MET in papillary RCC and collecting duct carcinoma might be helpful in discriminating from the other subtypes of RCC with tubulo-papillary growth. In clear cell RCC, there was a significant relationship between MET expression and high nuclear grade, as well as several clinicopathological parameters including infiltrative growth, tumoral necrosis, pelvic or ureteral involvement, calyceal involvement, and lymphatic invasion, implying invasiveness or aggressiveness of the tumor.
Figures and Tables
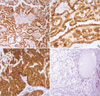 | Fig. 1Immunohistochemical staining for MET shows strong reactivity in the cell membrane with cytoplasmic staining in papillary renal cell carcinoma (A, ×200), collecting duct carcinoma (B, ×400) and urothelial carcinoma of renal pelvis (C, ×400). No reactivity was found in 55% of clear cell renal cell carcinomas (D, ×200). |
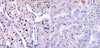 | Fig. 2Immunohistochemical staining for MET shows focal and weak reactivity in the cell membrane with or without cytoplasmic staining in chromophobe renal cell carcinoma (A, ×400). Focal and weak cytoplasmic staining is observed in less than 5% of tumor cells in 11 cases of renal oncocytoma (B, ×400). |
References
1. Gonzatti-Haces M, Seth A, Park M, Copeland T, Oroszlan S, Vande Woude GF. Characterization of the TPR-MET oncogene p65 and the MET protooncogene p140 protein-tyrosine kinases. Proc Natl Acad Sci USA. 1988. 85:21–25.

2. Rubin JS, Chan AM, Bottaro DP, Burgess WH, Taylor WG, Cech AC, Hirschfield DW, Wong J, Miki T, Finch PW, Aaronson SA. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci USA. 1991. 88:415–419.

3. Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987. 327:239–242.

4. Tsarfaty I, Resau JH, Rulong S, Keydar I, Faletto DL, Vande Woude GF. The met proto-oncogene receptor and lumen formation. Science. 1992. 257:1258–1261.

5. Woolf AS, Kolatsi-Joannou M, Hardman P, Andermarcher E, Moorby C, Fine LG, Jat PS, Noble MD, Gherardi E. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995. 128:171–184.

6. Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993. 123:223–235.

7. Horie S, Aruga S, Kawamata H, Okui N, Kakizoe T, Kitamura T. Biological role of HGF/MET pathway in renal cell carcinoma. J Urol. 1999. 161:990–997.

8. Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, Allikmets R, Chidambaram A, Bergerheim UR, Feltis JT, Casadevall C, Zamarron A, Bernues M, Richard S, Lips CJ, Walther MM, Tsui LC, Geil L, Orcutt ML, Stackhouse T, Lipan J, Slife L, Brauch H, Decker J, Niehans G, Hughson MD, Moch H, Storkel S, Lerman MI, Linehan WM, Zbar B. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997. 16:68–73.

9. Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, Lubensky I, Neumann HP, Brauch H, Decker J, Vocke C, Brown JA, Jenkins R, Richard S, Bergerheim U, Gerrard B, Dean M, Linehan WM, Zbar B. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999. 18:2343–2350.

10. Fischer J, Palmedo G, von Knobloch R, Bugert P, Prayer-Galetti T, Pagano F, Kovacs G. Duplication and overexpression of the mutant allele of the MET proto-oncogene in multiple hereditary papillary renal tumours. Oncogene. 1998. 17:733–739.
11. Sweeney P, EL-Naggar AK, Lin S, Pisters LL. Biological significance of c-met overexpression in papillary renal cell carcinoma. J Urol. 2002. 168:51–55.
12. The World Health Organization Working Group. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Tumours of the kidney. World Health Organization Classification. Pathology and Genetics of Tumours of the urinary system and male genital organs. 2004. Lyon: IARC Press;9–87.
13. Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997. 10:537–544.
14. Thoenes W, Störkel S, Rumpelt HJ, Moll R, Baum HP, Werner S. Chromophobe cell renal carcinoma and its variants-a report on 32 cases. J Pathol. 1988. 155:277–287.

15. American Joint Committee on Cancer. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. Genitourinary Sites. AJCC Cancer staging manual. 2002. 6th ed. Chicago: Springer;301–346.
16. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.

17. Inoue K, Karashima T, Chikazawa M, Iiyama T, Yoshikawa C, Furihata M, Ohtsuki Y, Shuin T. Overexpression of c-met proto-oncogene associated with chromophilic renal cell carcinoma with papillary growth. Virchows Arch. 1998. 433:511–515.

18. Pisters LL, el-Naggar AK, Luo W, Malpica A, Lin SH. C-met proto-oncogene expression in benign and malignant human renal tissues. J Urol. 1997. 158:724–728.

19. Natali PG, Prat M, Nicotra MR, Bigotti A, Olivero M, Comoglio PM, Di Renzo MF. Overexpression of the met/HGF receptor in renal cell carcinomas. Int J Cancer. 1996. 69:212–217.

21. Glukhova L, Lavialle C, Fauvet D, Chudoba I, Danglot G, Angevin E, Bernheim A, Goguel AF. Mapping of the 7q31 subregion common to the small chromosome 7 derivatives from two sporadic papillary renal cell carcinomas: Increased copy number and overexpression of the MET protooncogne. Oncogene. 2000. 19:754–761.
22. Jeffers M, Schmidt L, Nakaigawa N, Webb CP, Weirich G, Kishida T, Zbar B, Vande Woude GF. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc Natl Acad Sci USA. 1997. 94:11445–11450.

23. Lubensky IA, Schmidt L, Zhuang Z, Weirich G, Pack S, Zambrano N, Walther MM, Choyke P, Linehan WM, Zbar B. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol. 1999. 155:517–526.

24. Polascik TJ, Cairns P, Epstein JI, Fuzesi L, Ro JY, Marshall FF, Sidransky D, Schoenberg M. Distal nephron renal tumors: microsatellite allelotype. Cancer Res. 1996. 56:1892–1895.
25. Störkel S, Steart PV, Drenckhahn D, Thoenes W. The human chromophobe cell renal carcinoma: its probable relation to intercalated cells of the collecting duct. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989. 56:237–245.

26. Morra MN, Das S. Renal oncocytoma: a review of histogenesis, histopathology, diagnosis, and treatment. J Urol. 1993. 150:295–302.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download