Abstract
Cytokeratin 18 (CK18) protein was identified as an airway epithelial cell autoantigen associated with nonallergic asthma. Cleavage of CK18 protein by caspase-3 is a marker of early apoptosis in epithelial cells. It has been shown that the expression of active caspase-3 was increased in bronchial epithelial cells of asthmatic patients, when compared with healthy controls. To investigate the antigen-binding characteristics of IgG autoantibodies to CK18 protein in nonallergic asthma, the bindings of IgG autoantibodies to the fragments of CK18 protein cleaved by caspase-3 were analyzed by Western blot using serum samples from three patients with nonallergic asthma. Recombinant human CK18 protein was treated by caspase-3 and cleaved into N-terminal fragment (1-397 amino acids) and C-terminal fragment (398-430 amino acids). The binding capacity of IgG autoantibodies to N-terminal fragment of CK18 was maintained in one patient and reduced in other two patients. IgG autoantibodies from all three patients did not bind to C-terminal fragment of CK18. In conclusion, IgG autoantibodies to CK18 protein from patients with nonallergic asthma seems to preferentially bind to the whole molecule of CK18 protein and their antigen-binding characteristics were heterogeneous among the patients with nonallergic asthma.
Asthma is a chronic inflammatory disorder of airway with long history, even described by Hippocrates (1,2). Traditionally, asthma has been regarded as a clinical manifestation of allergic response to environmental agents (1). However, available epidemiological evidences suggest that the portion of asthma cases attributable to allergic sensitization (atopy) is less than one half (3). This indicates that the importance of etiological mechanism other than allergic mechanism might be neglected (3). This is especially true when considering the patients with severe asthma are more frequently nonallergic compared to those with mild-to-moderate asthma (4). The nonallergic asthma has been suggested as an autoimmune disease on the basis of frequent detection of circulating autoantibodies in patients with nonallergic asthma (1,5-7). However, a causal relationship between autoimmunity and asthma has not been demonstrated mainly due to lack of identification of pathogenetically and logically relevant autoantigen (8).
On a pathological viewpoint, airway epithelium has been suggested as a major target tissue for inflammatory response in asthma regardless of allergic sensitization (9) and could be a potential target of autoimmune response in asthma (10). We previously identified the cytokeratin 18 (CK18) protein as an airway epithelial autoantigen associated with nonallergic asthma while exploring the hypothesis that an autoimmune response to airway epithelial cell proteins might be involved in the pathogenesis of asthma (10).
Apoptosis and loss of adhesion of bronchial epithelial cells have been reported in patients with asthma and these have attributed to secretion of TNF-alpha and interferon gamma by T cells and eosinophils (11). Caspase-3 is a key mediator of apoptosis in mammalian cells and cleavage of CK18 protein by caspase-3 is a marker of early apoptosis in epithelial cells (12,13). It has been reported that the apoptotic caspase-3 protein was increased in bronchial epithelial cells of asthmatic patients, when compared with healthy controls (14).
In this study, the bindings of IgG autoantibodies to the fragments of CK18 protein cleaved by caspase-3 were analyzed by Western blot to investigate the antigen-binding characteristics of IgG autoantibodies in nonallergic asthma.
We used serum samples from three patients with nonallergic asthma who had circulating IgG autoantibodies against CK18 protein (Table 1). All patients had typical clinical history of asthma and documented reversibility of FEV1 greater than 15% after inhalation of bronchodilator. All patients underwent skin-prick test with 50 common aeroallergens (Bencard Co., Brentford, U.K.). Nonallergic asthma was defined when there was no positive skin reaction to any of the 50 common aeroallergens in the presence of a positive histamine control. All serum samples from subjects were aliquoted and stored at -20℃. All subjects gave informed consent, and the institutional review board approved this study.
Recombinant human CK18 protein (Research Diagnostics Inc., Pleasant Hill Road Flanders, NJ, U.S.A.) was separated by discontinuous sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS-PAGE) using an 8% resolving gel (pH 8.8) and a 4% stacking gel (pH 6.8). Following electrophoresis, proteins were transferred onto a polyvinylidine difluoride membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.). After the transfer, the membrane strips were probed with 1 mL of serum samples at dilution of 1 in 100 (v/v) for 2 hr at room temperature. After washes, the membrane was incubated with alkaline phosphatase-conjugated goat anti-human IgG (Sigma Chemical Co., St. Louis, MO, U.S.A.) for 2 hr at room temperature. After a final washing, the membrane was stained with a substrate solution (nitro blue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate; Sigma Chemical Co.). A mouse monoclonal antibody to human CK18 protein (clone no. CY-90, Sigma Chemical Co.) known to recognize a epitope located in the region of 312-356 amino acids of CK18 was used as a positive control (15). Alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma Chemical Co.) was used for detection of mouse monoclonal antibody.
1 µg of recombinant human cytokeratin 18 protein was incubated with 1 µg of caspase-3 (BD Biosciences, San Diego, CA, U.S.A.) in a 40 µL volume containing 50 mM HEPES, 0.1 M NaCl, 10% sucrose, 0.1% CHAPS, and 10 mM DTT for 4 hr at 37℃ and the reaction was stopped by adding SDS-PAGE sample buffer as described in a previous report (16).
Caspase 3 has been reported to cleave 394DALD/S site of CK18 protein (16) (Fig. 1). We observed degradation of 49-kDa recombinant human cytokeratin 18 protein into 45-kDa fragment (corresponding N-terminal 1-397 amino acids; visible in Fig. 2) and 4-kDa fragment (corresponding C-terminal 398-430 amino acids; invisible in protein staining due to small molecular weight of this fragment in Fig. 2).
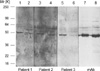
The binding capacity of IgG autoantibodies to N-terminal fragment (1-397 amino acid) of CK18 was maintained in one patient and reduced in other two patients (Fig. 3). IgG autoantibodies from all three patients did not show significant binding to C-terminal fragment (398-430 amino acids) of CK18 (Table 2). Mouse monoclonal IgG antibody to CK18 (clone CY-90; Sigma Chemical Co.) bound to the N-terminal fragment (1-397 amino acids) with same intensity of binding to whole molecule of CK18 protein (Fig. 3).
The presence of circulating IgG autoantibodies to CK18 could be just a reflection of epithelial damage in patients with nonallergic asthma considering recent evidence that the bronchial epithelium is more susceptible to injury in this group (17). Or the IgG autoantibodies could be a pathogenic factor contributing the development of chronic airway inflammation in nonallergic asthma.
Apoptosis is a biochemically and morphologically distinct form of programmed cell death, initiated by specific signals that activate specific caspases (13). As a result, the cell is eliminated by an intrinsic suicide program, resulting in DNA fragmentation, nuclear condensation, cytoskeletal reorganization, plasma membrane blebbing, and loss of cell adhesion (13). Apoptosis and loss of adhesion of bronchial epithelial cells have been reported in asthma and these have attributed to secretion of TNF-alpha and interferon gamma by T cells and eosinophils in asthmatic airway mucosa (11). Caspase inhibitor was shown to decrease airway inflammation in mouse model of asthma (18). Inhibition of epithelial cell apoptosis by corticosteroids has been suggested to be a mechanism of anti-asthmatic property of corticosteroids (19). Cleavage of CK18 protein by caspases and the exposure of specific CK18 neo-epitope identifiable by monoclonal antibody M30 (the same CK18 epitope exposed by caspase-3 in this study) has been reported to be a specific feature of early apoptosis (13). In previous study on idiopathic pulmonary fibrosis, the development of circulating IgG antibodies to cytokeratin 18 protein was suggested to be an secondary immune response to neo-epitopes of CK18 exposed by caspases during the apoptosis (20). In this study, we showed that IgG autoantibodies to cytokeratin 18 from patients with nonallergic asthma preferentially bound with whole molecule of CK18 protein, rather than the apoptotic fragments cleaved by caspase-3 and their antigen-binding characteristics were heterogeneous among the patients with nonallergic asthma. There is a certain limitation on the interpretation of these results due to the small number of patients (only three patients) because relatively large volumes of serum samples were needed for multiple repeated experiments. However, results of this study suggest that the autoantibody response to CK18 in nonallergic asthma might not be a secondary phenomenon associated with apoptotic death of airway epithelial cells due to chronic airway inflammation.
The idea of a possible involvement of autoimmunity in the pathogenesis of asthma has been proposed by earlier studies which demonstrated higher incidences of various autoantibodies against antigens in bronchial mucosa, paranasal sinus, lung, and endothelial cell in asthmatic patients compared to healthy controls (5-7). Analysis of T cell in airway fluid from patients with non-atopic asthma showed features of clonal expansion indicating the antigen-induced activation of T cells in the airway of non-atopic asthma (21). A significant correlation between T cell proliferative response to the 55-kDa endothelial autoantigen and the degree of airway obstruction has been reported in asthmatic patients (7). Patients with severe asthma can be clinically improved by anti-CD4 antibody treatment (22). In mouse model, non-atopic airway hyperresponsiveness could be passively transferred by CD4+ T cells (23). In rat model of autoimmune emphysema, transfer of pathogenic CD4+ T cells or antibodies developed by immunizing endothelial cell proteins caused emphysema (24). These suggest that a T cell response to an 'as-yet unidentified autoantigen' could be involved in the pathogenesis of nonallergic asthma (1,25). However, further studies including the T cell response to autoantigens and development of animal model for autoimmune asthma are necessary to determine the pathogenetic significance of autoimmunity in nonallergic asthma.
Figures and Tables
Fig. 1
Cleavage of cytokeratin 18 protein by caspase-3 at the sequence 394DALD/S (arrow). Cleavage of 49-kDa cytokeratin 18 protein (A) by caspase-3 results in 45-kDa fragment (B) and 4-kDa fragment (C).

Fig. 2
Protein stain of cytokeratin 18, caspase-3, and fragments of cytokeratin 18 treated by caspase-3. 0.25 µg of caspase-3 only (lane 1), 0.25 µg of cytokeratin 18 treated by 0.05 µg of caspase-3 (lane 2), molecular weight marker (lane 3), 0.25 µg of cytokeratin 18 only (lane 4), and 0.25 µg of cytokeratin 18 treated by 0.25 µg of caspase-3 (lane 5).
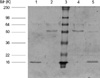
Fig. 3
Binding of IgG autoantibodies from three patients with nonallergic asthma to the fragments of cytokeratin 18 protein treated by caspase-3. Lane 1, 3, 5, and 7: 0.2 µg of cytokeratin 18 protein not treated by caspase. Lane 2, 4, 6, and 8: 0.2 µg of cytokeratin 18 protein treated by 0.2 µg of caspase-3. mAb: mouse monoclonal antibody to human cytokeratin 18 (clone no. CY-90, Sigma Co., St. Louis, MO, U.S.A.) known to recognize an epitope located in the region of 312-356 amino acids of cytokeratin 18 protein.
References
1. Global Initiative for Asthma: Global strategy for asthma management and prevention. 2002. Bethesda, Md: National Institutes of Health;50–66. (NIH publication no. 02-3659.).
2. Keeney EL. The history of asthma from Hippocrates to Meltzer. J Allergy Clin Immunol. 1964. 35:215–226.

3. Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy. Thorax. 1999. 54:268–272.

4. The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003. 22:470–477.
5. Wagner V, Tomšíková A, Šach J, Janková J, Novácková D. Autoimmune mechanism in diseases of the respiratory tract. Acta Allergol. 1965. 20:1–9.

6. Girard JP, Dami J. Antigenic properties of lung tissue. Presence of specific antibodies in certain chronic respiratory disorders. Poumon Coeur. 1973. 29:267–270.
7. Lassalle P, Delneste Y, Gosset P, Gras-Masse H, Wallaert B, Tonnel AB. T and B cell immune response to a 55-kDa endothelial cell-derived antigen in severe asthma. Eur J Immunol. 1993. 23:796–803.

8. Rottem M, Shoenfeld Y. Asthma as a paradigm for autoimmune disease. Int Arch Allergy Immunol. 2003. 132:210–214.

9. Montefort S, Herbert CA, Robinson C, Holgate ST. The bronchial epithelium as a target for inflammatory attack in asthma. Clin Exp Allergy. 1992. 22:511–520.

10. Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, Kim JK. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002. 165:1536–1539.

11. Trautmann A, Kruger K, Akdis M, Muller-Wening D, Akkaya A, Brocker EB, Blaser K, Akdis CA. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005. 138:142–150.

12. Grutter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000. 10:649–655.
13. Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999. 187:567–572.

14. Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, Mechighel P, Megret J, Leseche G, Aubier M, Pretolani M. Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J Respir Crit Care Med. 2001. 164:1487–1494.
15. Ditzel HJ, Strik MC, Larsen MK, Willis AC, Waseem A, Kejling K, Jensenius JC. Cancer-associated cleavage of cytokeratin 8/18 heterotypic complexes exposes a neoepitope in human adenocarcinomas. J Biol Chem. 2002. 277:21712–21722.

16. Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997. 138:1379–1394.
17. Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000. 14:1362–1374.

18. Iwata A, Nishio K, Winn RK, Chi EY, Henderson WR Jr, Harlan JM. A broad-spectrum caspase inhibitor attenuates allergic airway inflammation in murine asthma model. J Immunol. 2003. 170:3386–3391.

19. Wen LP, Madani K, Fahrni JA, Duncan SR, Rosen GD. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997. 273:L921–L929.
20. Dobashi N, Fujita J, Murota M, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ueda R, Bandoh S, Kamei T, Nishioka M, Ishida T, Takahara J. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung. 2000. 178:171–179.

21. Umibe T, Kita Y, Nakao A, Nakajima H, Fukuda T, Yoshida S, Sakamaki T, Saito Y, Iwamoto I. Clonal expansion of T cells infiltrating in the airways of non-atopic asthmatics. Clin Exp Immunol. 2000. 119:390–397.

23. De Sanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. T-lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med. 1997. 3:460–462.

24. Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005. 171:734–742.

25. Nahm DH. Pathogenetic mechanism of nonallergic asthma: autoimmune hypothesis. Korean J Asthma Allergy Clin Immunol. 2004. 24:69–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download