Abstract
Despite advances in the characterization of anaplastic large cell lymphoma (ALCL), little data is available on Asian patients. We report here upon single Korean institution's experience regarding the clinical characteristics and outcomes of ALCL. We performed a retrospective study of 32 adults with ALCL. Most of the patients received anthracycline-based chemotherapy. Ann Arbor stage III-IV, B symptoms, high-intermediate/high International Prognostic Index (IPI), and extranodal disease at diagnosis were present in 56%, 44%, 41%, and 63%, respectively. Compared with Western studies, the male/female ratio (4.3) was markedly higher and skin (9%) and bone involvement (9%) were less frequent. The staining results for anaplastic lymphoma kinase were positive in 6 (33%) of 18 cases available. The complete response (CR) rate was 62% (95% CI, 44-80%). With a median follow-up of 51.0 months, 5 yr overall survival was 40±11%. The 3 yr relapse-free survival for the 18 patients who achieved CR was 74±12%. Age, performance status, lactate dehydrogenase, extranodal disease sites number, and IPI were correlated with treatment response and survival. Our data suggest that Korean ALCL patients appear to have a higher male/female ratio, less frequent skin/bone involvement, and lower CR rate compared with those of Western studies.
Peripheral T-cell lymphomas (PTCL) are relatively rare in Western countries, and account for only 10 to 15% of all non-Hodgkin's lymphomas (NHL's) (1,2). Compared with Western countries, regions in Asia such as Hong Kong, Japan, and China have higher rates of PTCL (3). In Korea, PTCL constitute approximately 25 to 35% of all NHL's (4,5). PTCL consist of a variety of uncommon and rare entities. One of the predominantly nodal PTCL, anaplastic large cell lymphoma (ALCL) is heterogeneous in nature in terms of its clinical, morphologic, and cytogenetic features. Clinically, ALCL can be subdivided into primary (systemic and cutaneous) and secondary (anaplastic transformation from another lymphoma) forms, and primary systemic ALCL accounts for about 5% of all NHL's in adults (2,6). ALCL was found to be associated with the t(2;5) translocation (7), which results in the expression of anaplastic lymphoma kinase (ALK) protein. Moreover, there is an increasing evidence that the clinical features and the outcome of systemic ALCL are significantly dependent on ALK expression (8,9). The heterogeneity of the clinical features and outcomes of ALCL in different studies is probably due to differences in diagnostic criteria and age distributions, and to the inclusion (in addition to cases of systemic ALK-positive ALCL) of a variety of unrelated entities with a different prognosis, such as primary cutaneous ALCL and ALK-negative systemic ALCL.
Despite recent advances in the characterization of ALCL, its clinical outcome and optimal treatment have not clearly determined. In Korea, ALCL constitutes approximately 1.5 to 1.9% of NHL (4,5). We report here upon Korean experience concerning the clinical characteristics and treatment outcome of ALCL patients at a single institution.
The medical records of 32 consecutive adult patients with ALCL CD30+ (Ki-1), older than 16 yr were reviewed. All patients had been newly diagnosed between March 1993 and May 2003 at the Seoul National University Hospital. Histopathologic material was reviewed by one pathologist. A diagnosis of ALCL was made according to the standard diagnostic criteria detailed in the World Health Organization (WHO) classification for NHL (10), which includes classic histologic features and tumor cell CD30 reactivity. The panel of monoclonal antibodies used included CD45, CD30, CD15, CD20, CD79a, CD3, CD45RO, epithelial membrane antigen, Ki-67, CD56, and CD68.
Staging procedures included a physical examination, chest radiography, abdominal computerized tomography (CT) scan, a complete blood count, bilateral bone marrow aspirates and biopsies, liver and renal function tests, and lactate dehydrogenase (LDH) determinations. Staging was performed according to the Ann Arbor staging systems for Hodgkin's disease(11).
Chemotherapy regimens consisted of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) (12), COPBLAM-V (vincristine, bleomycin, cyclophosphamide, doxorubicin, prednisolone, procarbazine) (13), CVP (cyclophosphamide, vincristine, prednisolone), BVP (bleomycin, vincristine, prednisolone), IMEP (ifosfamide, methotrexate, etoposide, prednisolone), and CAPPE/VBM (cyclophosphamide, doxorubicin, prednisolone, procarbazine, etoposide, vincristine, bleomycin, methotrexate), and chemotherapy was administered for a total of 6 cycles. One month after therapy completion, re-staging was performed by physical examination, blood cell counts, liver and renal function tests, LDH evaluation, CT scans of abdomen, chest radiography, and by bone marrow biopsy, in cases showing tumor involvement at diagnosis. Assessment of treatment response was categorized as;complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the WHO criteria (14).
Overall survival (OS) was measured from the date of diagnosis to the date of death from any cause or the date of the last follow-up evaluation. Relapse-free survival (RFS) duration was calculated for CR patients from the date of CR until documented relapse, death or the last follow-up date in remission. Survival rates were estimated using the Kaplan and Meier method (15) and were compared using the log-rank test.
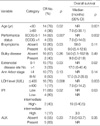
The main clinical findings of the 32 ALCL patients are shown in Table 1. Twenty-six patients (81%) were male and 6 (19%) female. Median age was 51 yr (range, 16-80 yr). Sixty-three percent of patients showed peripheral lymph node involvement. Advanced disease stage (Ann Arbor stage III-IV), B symptoms, and extranodal disease at diagnosis were present in 56%, 44%, and 63% of cases, respectively. The frequencies of extranodal sites of lymphoma involvement were as follows: liver (19%), lung (9%), pleural cavity (9%), peritoneal cavity (9%), skin (9%), and bone (9%), with involvement of bone marrow (6%) or gut (6%) being uncommon. The central nervous system (CNS) was affected in one patient (3%). Patients in high-intermediate/high risk group (International Prognostic Index [IPI] 3-5) constituted 41% of the cases.
Immunohistochemistry showed CD30+ in all cases. Phenotype was considered to be T-cell in 26 cases (81%) and null in 6 cases (19%). Staining results for ALK were available in 18 cases and 6 cases (33%) were ALK positive.
Thirty of the 32 patients were treated with combination chemotherapy: 15 (50%) with CHOP, 11 (37%) with COPBLAM-V, and 4 (13%) with other regimens (CVP, BVP, CAPPE/VBM, IMEP). Twenty-nine patients were assessable for response. After chemotherapy, the CR rate was 62% (18/ 29; [95% confidence interval (CI), 44-80%]) and the PR rate was 24% (7/29), with an overall response rate (CR+PR) of 86%; 14% (4/29) showed no response. Five out of 18 CR patients after chemotherapy were treated by involved-field radiation therapy due to initial bulky disease.
Four patients (22%) among 18 that achieved CR relapsed between 2.9 and 36.2 months after chemotherapy completion median, 5.1 months) and a second remission was achieved in 3 of these 4 by salvage chemotherapy, but the second remission duration was short (median, 2.6 months). Treatments for relapses were rather heterogeneous: 3 patients received IMEP and 1 patient DHAP (dexamethasone, cytarabine, prednisolone).
At the time of this analysis, the median follow-up period was 51.0 months (range, 11.6-135.9 months). The 5 yr OS for all 30 patients that received chemotherapy was 40±11% and the median OS was 54.0 months (95% CI, 0.8-107.2 months) (Fig. 1). The median OS for patients that achieved CR had not been reached and the 5 yr survival rate was 78±15%. In contrast, in patients who failed to achieve CR, mediansurvival was only 7.0 months (95% CI, 2.1-11.9 months).Median RFS for the 18 patients who achieved CR had notbeen reached and the 3 yr RFS was 74±12% (Fig. 2).
Responses to chemotherapy by pretreatment characteristics are detailed in Table 2. Age, performance status, LDH level, extranodal disease sites number, and IPI were found to be correlated with treatment response and with survival by univariate analysis. And, B symptoms were found to be correlated with response to treatment. IPI, which was developed for aggressive lymphomas in general, predicted survival in our group of patients (Fig. 3). The CR rates of the low, low-intermediate, high-intermediate, and high IPI risk groups were 85%, 80%, 40%, and 17% (p=0.02), respectively. Median OS was not reached in the low and low-intermediate IPI risk group, but was 19.0 months and 3.0 months in the high-intermediate and high risk group, respectively (p=0.03) (Table 2). When grouped into 2 categories (low/low-intermediate and high-intermediate/high IPI risk groups), the CR rates of the low/low-intermediate and high-intermediate/high IPI risk groups were 83% and 27%, respectively (p=0.005). Median OS was not reached in the low/low-intermediate IPI risk group, but was 9.0 months (95% CI, 0-24.1 months) in the high-intermediate/high risk group (p=0.001).
Meanwhile, no significant differences were found between the CR rate and survival of ALK-positive and ALK-negative cases; The CR rates of ALK-positive and ALK-negative ALCL cases were 83% and 55%, respectively (p=0.23) and their respective 5 yr survival rates were 60% and 25% (p=0.39).
Current knowledge of ALCL is limited because of the small numbers of patients analyzed in single series and the heterogeneity of treatments administered (16-21). Moreover, this is the first report to describe the clinical features and treatment outcomes of Korean ALCL patients.
Our study confirms the male predominance in ALCL (male: female, 26:6) and the young age of onset of ALK-positive ALCL (median age, 17 yr), as reported by others (8). The male/female ratio (4.3), however, was markedly higher than those found in Western studies (1.4-1.8) (19,20). ALCL patients in this study usually presented with enlarged peripheral, abdominal, or mediastinal nodes (84%). Similarly to other studies (16-20), ALCL patients in this study frequently presented with advanced disease (stage III-IV) (56%), B symptoms (44%), extranodal disease at diagnosis (63%), and high-intermediate or high risk IPI (41%). Extranodal sites were the liver (19%), lung (9%), pleural cavity (9%), peritoneal cavity (9%), skin (9%) and bone (9%). Bone marrow (6%), gut (6%), and CNS (3%) involvement were uncommon events. It appears that skin and bone involvement in our study are less frequent than those found in Western studies (8,16). However, the small number of patients in our study prevents our drawing firm conclusions in terms of clinical presentations. Most of the clinical features except for gender ratio and extranodal involvement pattern in Korean ALCL patients in the present study are similar to those reported in the West (16-20) and other Asian countries (22,23), though there are few data in Asians.
Despite recent advances in the characterization of ALCL, its rarity has belied the establishment of an optimal treatment. Although no large comparative studies have been published, most investigators have reported that response to chemotherapy is good, ranging from 60% to 90% in ALCL (16-21). Most patients (90%) in the present study received anthracycline-based chemotherapy regimens. CR rate was 62% (95% CI, 44-80%), and the 5 yr OS for all 30 patients that received chemotherapy was 40±11% and the median OS was 54.0 months. Three-year RFS of the 18 patients who achieved CR was 74±12%. In the literature, OS and disease-free survival for ALCL treated by chemotherapy ranges from 29% to 77% and from 50% to 67%, respectively (16-21). Although the outcomes in the present study may be less informative because of various treatment regimens, the CR rate of 62% and 5 yr survival rate of 40% was at the lower limit of what has been reported in the West (16-21).
There is now evidence that the clinical features and outcomes of systemic ALCL differ significantly for cases harboring or lacking ALK expression (8,9,24). In our study, the expression of ALK protein was confirmed retrospectively by immunohistochemistry in 6 of 18 cases (33%) examined. The ALK expression rate in our study appears to be lower than the 50-60% reported in the literature (8,9,25), however, small number of cases makes it difficult to draw a firm conclusion.
In the present study no significant differences were found between the clinical features or survivals of ALK-positive and ALK-negative cases, which is not surprising given the small number of ALK cases available (n=18). ALK-positive ALCL more frequently presented with aggressive stage III to IV disease (50% vs. 33%), extranodal involvement (83% vs. 42%), and low/low-intermediate IPI (83% vs. 58%), and had high CR rate (83% vs. 55%) and 5 yr survival rate (60% vs. 25%), but all were without statistical significance. For further evaluation of the significance of ALK in Korean ALCL patients, a larger national or multi-institutional study is needed.
In addition to ALK-positivity, IPI has been identified as an important factor for predicting prognosis in ALCL by several studies (9,26,27). In patients with ALK-positive lymphomas, the 5 yr survival rate was 94±5% in the low- to intermediate-risk group (age adjusted IPI: 0-1) and 41±12% in the high- to intermediate-risk group (age adjusted IPI: ≥2) (8). In cases of ALK-negative ALCL, IPI was also found to be a significant prognostic factor of OS (28). Similarly, in our study, IPI was correlated with treatment response and survival by univariate analysis. High-intermediate/high risk group had lower CR rate and shorter survival than low/low-intermediate risk group. IPI may be more useful in ALK-negative systemic ALCL in which the clinical outcome is highly variable and more difficult to predict in individual cases (26,29).
ALCL patients have better survival than those with other forms of PTCL (30,31). However, this relatively favorable prognosis of ALCL can be attributed to the subgroup with ALK expression. ten Berge et al. (28) reported no differences in overall and progression-free survival in ALK-negative ALCL and PTCL-NOS (not otherwise specified). Therefore, future treatment strategy in ALCL need to be stratified according to known risk factors, such as, ALK-negativity and high/high-intermediate IPI.
In conclusion, our data suggest that Korean ALCL patients appear to have a higher male/female ratio, less frequent skin and bone involvement, and lower CR rate to anthracycline-based chemotherapy compared with those found in Western studies. These, however, need to be further investigated in larger national or multi-institutional study. Further treatment study stratified according to risk factors such as ALK expression and IPI would contribute to the establishment of an optimal treatment for ALCL.
Figures and Tables
 | Fig. 1Overall survival of the 30 patients with ALCL who received chemotherapy (median 54.0 months; 5 yr survival rate 40±11%). |
 | Fig. 2Relapse-free survival for the 18 patients who achieved complete remission (3 yr relapse-free survival rate 74±12%). |
 | Fig. 3Overall survival according to International Prognostic Index in the 30 patients who received chemotherapy. The median survival of low and low-intermediate group was not reached and that of high-intermediate and high risk groups was 19.0 months and 3.0 months, respectively (p=0.03). |
References
1. Melnyk A, Rodriquez A, Pugh WC, Cabanillas F. Evaluation of the revised European-American lymphoma classification confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin's lymphoma. Blood. 1997. 89:4514–4520.

2. The Non-Hodgkin's lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group Classification of non-Hodgkin's lymphoma. Blood. 1997. 89:3909–3918.
3. Shih LY, Liang DC. Non-Hodgkin's lymphomas in Asia. Hematol Oncol Clin North Am. 1991. 5:983–1001.

4. Ko YH, Kim CW, Park CS, Jang HK, Lee SS, Kim SH, Ree HJ, Lee JD, Kim SW, Huh JR. REAL classification of malignant lymphomas in the Republic of Korea. Cancer. 1998. 83:806–812.

5. Kang YK, Kim BS, Kim TW, Ryu MH, Lee SS, Ryoo BY, Kim TY, Im YH, Lee KH, Huh J, Heo DS, Bang YJ, Kim C, Lee JS, Kim BK, Kim WK, Kim SH, Kim NK. Clinicopathologic characteristics of Korean non-Hodgkin's lymphomas based on REAL classification. J Korean Cancer Assoc. 1999. 31:641–652.
6. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink HK, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994. 84:1361–1392.
7. Rimokh R, Magaud JP, Berger F, Samarut J, Coiffier B, Germain D, Mason DY. A translocation involving a specific breakpoint (q35) on chromosome 5 is characteristic of anaplastic large cell lymphoma (Ki-1 lymphoma). Br J Haematol. 1989. 71:31–36.

8. Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, Verhoef G, Menestrina F, Todeschini G, Paulli M, Lazzarino M, Giardini R, Aiello A, Foss HD, Araujo I, Fizzotti M, Pelicci PG, Flenghi L, Martelli MF, Santucci A. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999. 93:2697–2706.
9. Gascoyne RD, Aoun P, Wu D, Chhanabhai M, Skinnider BF, Greiner TC, Morris SW, Connors JM, Vose JM, Viswanatha DS, Coldman A, Weisenburger DD. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood. 1999. 93:3913–3921.

10. Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. 2001. Lyon: IARC Press.
11. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971. 31:1860–1861.
12. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA Jr, Miller TP. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993. 328:1002–1006.

13. Coleman M, Armitage JO, Gaynor M, McDermott D, Weisenburger DD, Adler K, Beshevkin M, Silver RT, Reisman AM, Pasmantier MW. The COPBLAM programs: Evolving chemotherapy concepts in large cell lymphoma. Semin Hematol. 1988. 25:23–33.
14. World Health Organization. WHO offset publication no. 48. WHO handbook for reporting results of cancer treatment. Geneva, Switzerland: World Health Organization.
15. Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958. 53:457–481.
16. Shulman LN, Frisard B, Antin JH, Wheeler C, Pinkus G, Magauran N, Mauch P, Nobles E, Mashal R, Canellos G. Primary Ki-1 anaplastic large-cell lymphoma in adults: Clinical characteristics and therapeutic outcome. J Clin Oncol. 1993. 11:937–942.

17. Zinzani PL, Bendandi M, Martelli M, Falini B, Sabattini E, Amadori S, Gherlinzoni F, Martelli MF, Mandelli F, Tura S, Pileri SA. Anaplastic large-cell lymphoma: Clinical and prognostic evaluation of 90 adult patients. J Clin Oncol. 1996. 14:955–962.

18. Clavio M, Rossi E, Truini M, Carrara P, Ravetti JL, Spriano M, Vimercati AR, Santini G, Canepa L, Pierri I, Celesti L, Miglino M, Castellaneta A, Damasio E, Gobbi M. Anaplastic large cell lymphoma: A clinicopathologic study of 53 cases. Leuk Lymphoma. 1996. 22:319–327.
19. Longo G, Fiorani C, Sacchi S, Callea V, Lombardo M, Federico M, Stelitano C, Angrilli F, Vallisa D, Gobbi PG, Ilariucci F, Frassoldati A, Petrini M, Silingardi V. Clinical characteristics, treatment outcome and survival of 36 adult patients with primary anaplastic large cell lymphoma. Haematologica. 1999. 84:425–430.
20. Tilly H, Gaulard P, Lepage E, Dumontet C, Diebold J, Plantier I, Berger F, Symann M, Petrella T, Lederlin P, Briere J. Primary anaplastic large-cell lymphoma in adults. Clinical presentation, immunophenotype, and outcome. Blood. 1997. 90:3727–3734.

21. Pileri S, Bocchia M, Baroni CD, Martelli M, Falini B, Sabattini E, Gherlinzoni F, Amadori S, Poggi S, Mazza P. Anaplastic large cell lymphoma (CD30+/Ki-1+): results of a prospective clinico-pathological study of 69 cases. Br J Haematol. 1994. 86:513–523.

22. Lee SC, Kueh YK, Lehnert M, Chong SM, Tan YO, Wong J. Characteristics and prognosis of KI-1 positive anaplastic large cell lymphoma in Asians. Aust N Z J Med. 1998. 28:790–794.

23. Lin CN, Hou CC, Hwang WS, Chuang SS. Anaplastic large cell lymphoma-a rare disorder in southern Taiwan. Leuk Lymphoma. 2003. 44:1727–1731.

24. Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, Suzuki R, Kagami Y, Ogura M, Morishima Y, Mizoguchi Y, Okamoto M, Seto M, Koshikawa T, Mori S, Suchi T. Anaplastic large cell lymphoma: a distinct molecular pathologic entity. A reappraisal with special reference to p80NPM/ALKexpression. Am J Surg Pathol. 1997. 21:1420–1432.
25. Kinney MC, Kadin ME. The pathologic and clinical spectrum of anaplastic large cell lymphoma and correlation with ALK gene dysregulation. Am J Clin Pathol. 1999. 111:Suppl 1. 56–67.
26. ten Berge RL, Dukers DF, Oudejans JJ, Pulford K, Ossenkoppele GJ, de Jong D, Misere JF, Meijer CJ. Adverse effects of activated cytotoxic T-lymphocytes on the clinical outcome of nodal anaplastic large cell lymphoma. Blood. 1999. 93:2688–2696.
27. ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Pulford K, Willemze R, Falini B, Chott A, Meijer CJ. ALK expression in extranodal anaplastic large cell lymphoma favours systemic disease with (primary) nodal involvement and a good prognosis and occurs before dissemination. J Clin Pathol. 2000. 53:445–450.
28. ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJ. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003. 43:462–469.

29. Suzuki R, Kagami Y, Takeuchi K, Kami M, Okamoto M, Ichinohasama R, Mori N, Kojima M, Yoshino T, Yamabe H, Shiota M, Mori S, Ogura M, Hamajima N, Seto M, Suchi T, Morishima Y, Nakamura S. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood. 2000. 96:2993–3000.
30. Lopez-Guillermo A, Cid J, Salar A, Lopez A, Montalban C, Castrillo JM, Gonzalez M, Ribera JM, Brunet S, Garcia-Conde J, Fernandez de Sevilla A, Bosch F, Montserrat E. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the REAL classification. Ann Oncol. 1998. 9:849–855.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download