Abstract
Mesenchymal hamartoma (MH) of the liver is an uncommon benign lesion related to ductal plate malformation. It is usually cystic and mainly composed of myxoid mesenchymal tissue with tortuous or cystic bile ducts. In order to characterize the clinicopathological features of MH, the Korean Gastrointestinal Pathology Study Group collected a total of 17 MH cases diagnosed in 7 hospitals from 1992 to 2002 and compared the clinicopathologic findings of cystic MH with those of solid variant. Among the 17 cases, 7 (41%) were solid. The solid form showed a higher serum level of α-fetoprotein (AFP), the smaller bile ducts, and more frequent proliferation of vessels. Serum AFP level was related to the amount of hepatocytes. Two of seven solid cases harbored a larger amount of evenly distributed hepatocytes and proliferation of small duct with focal hepatocyte-bile duct transition. These histologic findings are similar to those of mixed hamartoma. Therefore, the mixed hamartoma and the MH of both solid and cystic types could be the variants of one disease spectrum. And hepatocytes within MH might be rather a genuine tumor component than entrapped into the tumor. In conclusion, MH can show various clinicopathological features and recognition of these features will facilitate accurate diagnosis of MH.
Mesenchymal hamartoma (MH) of the liver is a rare benign lesion mostly affecting children particularly in the first 2 yr of life (1), but a few cases have been reported in adults (2). It is frequently cystic, and consists mainly of mesenchymal stroma and architecturally abnormal biliary structures with hepatocyte lobules as a minor component (1, 2). The pathogenesis of MH has been considered to be aberrant development of primitive mesenchyme in the portal tract (3). However, some recent evidences have raised the possibility of true neoplasm (4-6) and those are as follows: the balanced translocation involving chromosome 19q13.4 (4); rare cases of malignant transformation (5); and aneuploidy in flow cytometric analysis (6).
The cystic nature of MH is the result of cystic degeneration of myxoid stroma or cystic dilatation of bile ducts (7). Rarely, solid form of MH containing a larger amount of hepatocytes has been documented (8, 9). As for the origin of the hepatocytes within MH, it has been proposed that they might be entrapped from the surrounding hepatic lobules or the actual tumor component aberrantly derived by extension of the derivatives of the primitive hepatic diverticulum growing into the septum transversum (3).
In contrast to the typical cystic MH, the clinicopathologic findings of the solid variant of MH have not been well characterized. Herein, we summarized 17 MHs including 10 cystic cases and 7 solid variants, and compared the clinicopathologic features of the solid variant with those of the typical cystic cases.
We collected 17 cases of MH of the liver diagnosed in 7 hospitals (Seoul National University, Seoul; Sungkyunkwan University, Seoul; University of Ulsan, Seoul; Yonsei University, Seoul; Chungnam National University, Daejeon; Kyungpook National University, Daegu; and Chonbuk National University, Chonju, Korea) from 1 January, 1992 to 31 December, 2002. Originally, twenty-five cases had been diagnosed as MH, but eight cases were excluded according to the result of microscopic revision. The revised diagnosis was infantile hemangioendothelioma in 3 cases, focal nodular hyperplasia in 2 cases, polycystic liver disease in 2 cases, and angiomyolipoma in 1 case. The diagnostic criteria for MH were based on the overgrowth of mesenchymal stroma and the proliferation of the architecturally abnormal bile ducts with or without cystic change, accompanied by periductal collaring of stromal cells (2, 3). In order to confirm the original diagnosis and to evaluate the relevant pathologic findings, eight pathologists reviewed the representative glass slides of all cases. The gender, age and serum α-fetoprotein (AFP) level of patients, and size and location of tumor were evaluated by a review of the medical charts and the pathologic records.
A variety of pathologic findings evaluated were as follows: the gross finding of MH (cystic or solid) (Fig. 1); the amount (moderate or abundant) and the nature (myxoid, collagenous or hyalinized) of mesenchymal stroma; the presence or absence of duct proliferation and the architecture of bile duct (cystic, tortuous, or small); the amount of hepatocytes (little or moderate); and the presence or absence of vascular proliferation. The amounts of stroma and hepatocytes were semiquantitatively assessed: little in <10%, moderate in 10-50%, abundant in >50% of the MH.
Comparison of these various pathologic findings between cystic and solid MHs were statistically analyzed using the Mann-Whitney U-test and t-test. A p value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS-PC software (SPSS, Chicago, IL, U.S.A.).
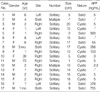
Clinical data of 17 cases were summarized in Table 1. There were 9 females and 8 males. The mean age at diagnosis was 9 yr, ranging from 1 month to 73 yr. Eleven out of 17 patients (65%) were 2 yr of age or younger and two patients were adult, 39 and 73 yr old. The MH involved the right lobe of liver in 10 out of 17 cases (59%), the left lobe in 4 cases (23%) and both lobes in 3 cases (18%). The majority of the patients (15 out of 17, 88%) had solitary while two cases had multiple tumors. The mean diameter of MH was 11.4±8.8 cm (range 2 to 20 cm). In case No. 11 (Table 1), a small MH, measuring 2 cm in diameter, was incidentally detected in the liver of a 79-yr-old man during an emergent operation for diaphragmatic rupture due to traffic accident. It was located in the right lobe of liver and was multicystic showing irregularly dilated bile ducts in the fibrous stroma (Fig. 2A).
Serum AFP had been checked in 13 patients and found to be elevated in 6 patients, but the AFP elevation in two infant patients (case No. 8 and No.17) might be physiologic, since the AFP level do not reach the adult level until 8 months of age (normal: less than 30 ng/mL at 1 yr of age, less than 15 ng/mL in adult) (10, 11).
As the common finding of all MHs, a moderate to abundant amount of mesenchymal stroma containing numerous bile duct structures of abnormal configuration accompanying periductal collaring of stromal cells constituted the mass (Fig. 2B). The stroma was myxoid in 9 out of 17 cases (53%) (Fig. 3A) and collagenous or hyalinized in 8 cases (47%) (Fig. 3B). Bile ducts were cystic (Fig. 3C) or tortuous in 10 cases (59%) (Fig. 3D, E) and small in 7 cases (41%) (Fig. 3F). Cystic changes were various in the amount and size, and appeared in 13 cases (76%) as simply dilated bile ducts, pseudocysts or as lymphangiomatous structures.
As a minor component of MH, vessels and hepatocytes were identified. Vascular proliferation was present in 11 out of 17 cases (65%) and the vessels were small- to medium-sized veins or capillaries. Hepatocytes were found in cords, islands or lobules. In 10 cases (59%), proliferation of malformed bile ductule or small bile duct structure was identified focally at the periphery of hepatocytic islands or lobules, occasionally ingrowing into the latter (Fig. 4A). Transition between hepatocytes and bile ducts was also identified focally in 8 cases (47%) (Fig. 4B). The hepatocytes were largely located in the periphery of the hamartoma. However, in two cases (case No. 7 and No. 17), they were widely distributed within the entire hamartomas.
The amount of hepatocytes was positively correlated with the serum level of AFP (361.4±352.6 ng/mL in MH with moderate amount of hepatocytes vs. 4.2±1.3 ng/mL in MH with little amount of hepatocytes; p=0.031). The correlation between the amount of hepatocytes and the serum level of AFP was also significant in the cystic forms (p=0.025), as well as in the solid forms (p=0.046).
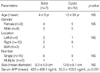
Ten cases (59%) were predominantly cystic, whereas 7 cases (41%) were predominantly solid. Clinical findings of cystic MH were not significantly different from those of solid MH, except serum AFP level (Table 2). The AFP level were significantly higher in patients with solid MH than in those with cystic MH (422±409.1 ng/mL vs. 55.5±103.5 ng/mL, respectively; p=0.031).
In cases of the cystic MH, the cysts were dilated bile ducts in 5 out of 10 cases (50%), pseudocysts secondary to cystic degeneration of mesenchymal stroma in 4 cases (40%) or lymphangiomatous with a lining of attenuated cells in 1 case (10%).
In the solid variant of MH, the bile ducts were more frequently small (71%), compared to those of the cystic MH (20%) (p=0.04), and vascular proliferation was also more frequently identified (100% vs. 40% respectively; p=0.013) (Table 3). Other pathologic findings including the amount and the nature of mesenchymal stroma, the ductular proliferation, and the amount of hepatocytes were not significantly different between the cystic and the solid MHs.
The term "mesenchymal hamartoma", now widely accepted, was coined by Edmondson in 1956, but before he introduced the term, it had been described under a variety of names: bile cell fibroadenoma, bile duct fibroadenoma, lymphangioma, cavernous lymphangiomatoid tumor, pseudocystic mesenchymal tumor, benign mesenchymoma, and cystic hamartoma (12). According to the original description by Edmondson, MH was a cystic mesenchymal lesion, chiefly composed of connective tissue containing much serous fluid (12). However, depending upon the amount and nature of stroma, it may present as a cystic or solid mass.
Stocker and Ishak described that MHs of the older patients were more commonly cystic and those of the younger patients more commonly solid (1), whereas all of 18 childhood cases in a 35-yr review of DeMaioribus et al. were cystic (7). In the present study, only two were the adult cases and both were cystic MH, while 7 out of 15 (47%) child cases were predominantly solid. These findings are in agreement with the description of Stocker and Ishak (1), although the cystic components were less frequent in the present study. In contrast to the cases of Cook et al. in which the stromal component of childhood MHs was fibrotic with areas of dense hyalinization and only focal myxoid areas (2), there was no significant difference in the stromal component between the adult and childhood cases and between the cystic and solid cases in our collection.
In the present study, some cases of solid MH showed features of the so-called "mixed hamartoma" including larger amount of hepatocyte, ductal proliferation and hepatocellular-ductal transition. The mixed hamartoma is a very rare hamartomatous lesion composed of all cellular components of the normal liver and it shares histologic features of vascularized fibroductular tissue with mesenchymal hamartoma and focal nodular hyperplasia (13, 14). Rhodes et al. described that mixed hamartoma could be distinguished from the ordinary cystic MH or focal nodular hyperplasia by the lack of cystic change, the presence of embryonal hepatocytes with hepatocellular-bile ductular transformation, and the presence of the extremely broad fields of ductules and central veins (13). However, the findings in the present series suggest that the mixed hamartoma and MH may be in one disease spectrum, since there are some overlapping in the histological findings of both hamartomas in addition to the common feature of periductal collaring of mesenchymal stroma. Both hamartomas are likely to be derived from portal tract (3, 13), but the mixed hamartoma is less differentiated and solid, whereas the MH is more differentiated and cystic (13). Chan et al. had suggested that the morphology of MH might be changed with age as follows: progressive loss of hepatocytes; degeneration of bile duct epithelium; and cystic change of the mesenchymal components (15). According to their suggestion, the mixed hamartoma and solid variant of MH may be the immature forms of the ordinary cystic MH.
It has been traditionally considered that the hepatocytes within MH are entrapped from the surrounding normal liver (3). However, when we consider the embryonal feature of hepatocytes in the mixed hamartoma and the positive correlation between serum AFP level and the amount of hepatocytes in solid MH of the present study, some hepatocytes in MH is suggested to be immature hepatocytes developed as a components of the lesion rather than entrapped mature hepatocytes.
The serum levels of AFP in patients with MH were measured in some previous studies where it was largely within normal limits (8, 9, 16, 17). But Ito et al. reported that five out of 7 Japanese cases of MH showed a high serum level of AFP ranging 3,200 to 6,000 ng/mL, and demonstrated the immunohistochemical localization of AFP in the proliferating hepatocytes and bile duct epithelium of MH (9). In the present study, the elevation of serum AFP level was found in 6 out of 13 cases (46%), and was more frequently noted in the predominantly solid MH than in the predominantly cystic form. This correlation may also support that the hepatocytes within the tumor mass are a true component of MH.
In conclusion, MH can show various clinicopathologic features and recognition of the clinicopathological differences between the typical MH and its variants will facilitate its accurate diagnosis.
Figures and Tables
Fig. 2
Typical histologic feature of MH. (A) Histologic finding of adult case of MH, incidentally found in a 79-yr-old man (case 11) (H&E, ×40). (B) Myxoid mesenchymal stroma and proliferation of the architecturally abnormal bile ducts with periductal collaring of stromal cells
(H&E, ×40).
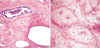
Fig. 3
Various histologic features of stroma and bile duct in MH. (A) Myxoid stroma (H&E, ×40). (B) Collagenous stroma (H&E, ×100). (C) Cystically dilated bile ducts (H&E, ×100). (D, E) Tortuous bile ducts (H&E, ×100 and ×200, respectively). (F) Small bile ducts (H&E, ×200).
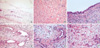
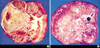
Fig. 4
Histologic finding of solid MH containing a larger amount of hepatocytes. (A) The bile ducts are ingrowing into hepatocytes (H&E, ×100). (B) Transition between hepatocytes and bile ducts is identified (H&E, ×200).

References
1. Stocker JT, Ishak KG. Mesenchymal hamartoma of the liver: report of 30 cases and review of the literature. Pediatr Pathol. 1983. 1:245–267.

2. Cook JR, Pfeifer JD, Dehner LP. Mesenchymal hamartoma of the liver in the adult: association with distinct clinical features and histological changes. Hum Pathol. 2002. 33:893–898.

3. Dehner LP, Ewing SL, Sumner HW. Infantile mesenchymal hamartoma of the liver. Histologic and ultrastructural observations. Arch Pathol. 1975. 99:379–382.
4. Rakheja D, Margraf LR, Tomlinson GE, Schneider NR. Hepatic mesenchymal hamartoma with translocation involving chromosome band 19q13.4: a recurrent abnormality. Cancer Genet Cytogenet. 2004. 153:60–63.

5. Ramanujam TM, Ramesh JC, Goh DW, Wong KT, Ariffin WA, Kumar G, Taib NA. Malignant transformation of mesenchymal hamartoma of the liver: case report and review of the literature. J Pediatr Surg. 1999. 34:1684–1686.

6. Abdulkader I, Fraga M, Perez-Becerra E, Reyes-Santias RM, Bautista A, Chaves E, Forteza J. Mesenchymal hamartoma of the liver; Clinicopathological, immunohistochemical and flow cytometric study of two cases. Hepatol Res. 2004. 28:216–219.

7. DeMaioribus CA, Lally KP, Sim K, Isaacs H, Mahour GH. Mesenchymal hamartoma of the liver. A 35-year review. Arch Surg. 1990. 125:598–600.
8. Sonobe H, Ohtsuki Y, Enzan H, Kurashige T, Matsuura K, Kaneko A, Ogata T. An unusual case of solid hamartoma in the liver. Acta Pathol Jpn. 1988. 38:75–82.

9. Ito H, Kishikawa T, Toda T, Arai M, Muro H. Hepatic mensenchymal hamartoma of an infant. J Pediatr Surg. 1984. 19:315–317.

10. Ohama K, Nagase H, Ogino K, Tsuchida K, Tanaka M, Kubo M, Horita S, Kawakami K, Ohmori M. Alpha-fetoprotein (AFP) levels in normal children. Eur J Pediatr Surg. 1997. 7:267–269.

11. Painter PC, Cope JY, Smith JI. Burtis CA, Ashwood ER, editors. Refence information for the clinical laboratory. Tietz textbook of clinical chemistry. 1999. Philadelphia: W.B. Saunders;1789–1840.
12. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956. 91:168–186.

13. Rhodes RH, Marchildon MB, Luebke DC, Edmondson HA, Mikity VG. A mixed hamartoma of the liver: light and electron microscopy. Hum Pathol. 1978. 9:211–221.

14. Toda T, Yamada M, Nakama B, Yamazato M, Hokama A, Muto Y. Immunohistochemical and electron microscopic findings in a case of mixed hamartoma of the liver. Jpn J Surg. 1990. 20:101–106.

15. Chau KY, Ho JW, Wu PC, Yuen WK. Mesenchymal hamartoma of liver in a man: comparison with cases in infants. J Clin Pathol. 1994. 47:864–866.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download