Abstract
The purpose of this study is to characterize and compare the ultrastructural changes occurring during the in vivo cultivation of corneal epithelium on amniotic membrane (AM) at several different time points. Corneal burn patients (n=7) with a corneal epithelial defect and severe limbal damage were selected. Initially, AM transplantation with limbal autograft was performed at the acute stage of corneal burn to reconstruct the damaged ocular surface. One to six (mean interval; 3.3±1.2) months later, the central part of AM containing an in vivo expanded corneal epithelium was excised and retransplanted in adjacent lesions. The excised epithelium with AM was examined by electron microscopy and immunohistochemical study. By electron microscopy, one and two months after expansion, cultivated epithelium on AM showed an undifferentiated epithelium and an incomplete basement membrane (BM). But, after three months, the cultivated epithelium began to differentiate into a multilayered epithelium with a continuous BM with increased hemidesmosomes. These findings were further confirmed by immunohistochemical study, that cytokeratin K3 was expressed in the cultivated corneal epithelium and newly formed BM was partially positive of collagen IV at three months. At least 3 months may be needed for the proliferation and differentiation of in vivo cultivated corneal epithelium on AM.
The ocular surface is covered by corneal and conjunctival epithelia; stem cells of the corneal epithelium are located exclusively at the limbus (1). Moreover, the integrity of limbal epithelial stem cells dictates the well-being and homeostasis of the corneal epithelium. When a severe corneal burn destroys both corneal and limbal epithelia, the cornea is healed in the pathologic state of limbal stem cell deficiency, which manifests as a persistent epithelial defect, conjunctivalization, and neovascularization, collectively leading to a severe visual loss (2).
In the past decades, surgical techniques for reconstructing the burned ocular surface have greatly advanced due to the introduction of limbal epithelial cell transplantation (3) and amniotic membrane transplantation (4,5). Several reports have shown that the treatment of severe ocular surface diseases by limbal transplantation in conjunction with amniotic membrane (AM) is often highly successful (6,7). Based on these encouraging results, we proposed transplanting limbal epithelium and AM at the same time to manage chemical burns at the acute stage, and have reported favorable outcomes (8).
However, in some severe cases, this combined transplantation cannot completely prevent cicatricial complications at a late stage. And, once cicatricial complications, such as pseudopterygium, have occurred, subamniotic membrane opacity begins to appear in the central portion of cornea, opacity then gradually increases with time and results in visual loss.
Thus, this subamniotic membrane opacity and pseudopterygium were removed simultaneously for visual rehabilitation. This procedure requires covering the epithelial and superficial stromal defects on peripheral cornea caused by pseudopterygium removal with a graft.
So far, 'ex vivo' corneal epithelium cultured in a laboratory has been mainly used for this purpose, and according to reports, clinical results have been successful (9,10). However, in order to use ex vivo cultivated corneal epithelium, extra time, additional tools and facilities for cultivation are necessary, and continuous management and special technology are needed during the cultivation. Therefore, it is not possible for many ophthalmologists to perform ocular surface reconstruction using ex vivo cultivated corneal epithelium.
In this study, the excised opacity, which was an in vivo expanded corneal epithelium on AM, looked healthy in integrity despite its opacity. Therefore, we used this excised opacity as a graft material for retransplantation onto epithelial defects.
In our previous works, we reported good clinical results obtained using this technique, and this serial procedure was referred to as a 'stepwise operation' (8). In this study, we addressed the question as to whether in vivo expanded corneal epithelium is clinically suitable as a graft material for ocular reconstruction, and if so, how much time is required after the first transplantation to prepare expanded corneal epithelium on AM? Here we report on an investigation of the corneal epithelial phenotype of the reconstructed tissue by electron microscopy and immunohistochemistry.
This study involved 7 eyes of 6 patients who had undergone conjunctival limbal autograft and amniotic membrane transplantation for acute chemical burns, which were graded as 3 or worse according to Hughes-Roper-Hall (11), and which developed pseudopterygium and corneal opacity as late complications at our university hospital from December 2000 through to May 2003. These 7 eyes suffered from alkali burns (2 eyes), acid burns (2 eyes), or thermal burns (3 eyes). Surgeries were approved by the institutional review board. Informed consent for the surgical procedures was obtained from each patient. Table 1 summarizes the cause of injury, burn grade, surgical procedures.
Abnormal corneal epithelium and superficial fibrovascular scar tissue were debrided by blunt dissection. A conjunctival peritomy was then performed 360° around the limbus. The AM was prepared as previously described (12) and then spread over the whole cornea with the basement membrane (BM) side up. An interrupted suture with 10-0 monofilament nylon anchored the AM graft to conjunctiva-episclera 2, 3 mm from the limbus. Transplantation of the conjunctival limbal autograft (CLAU) followed (excepting case 6). Briefly, conjunctival limbal tissue (2×6 mm) was removed from the patient's healthy contralateral eye and transplanted to the limbal area of the burned eye with interrupted sutures to anchor the graft to the underlying AM. Finally, a large piece of the AM was applied over the entire cornea as a temporary patch and removed 3 days later.
All of these 7 eyes subsequently developed pseudopterygium, neovascularization and central corneal opacity 1 to 6 months after the first procedure. We then removed the pseudopterygium and the central opacity (ca. 5-7 mm in diameter) including in vivo expanded corneal epithelium with the underlying AM. The in vivo expanded corneal epithelium together with AM was then retransplanted to the epithelial defect created by pseudopterygium removal in the peripheral cornea. The sizes of excised corneal epithelia were enough to cover the defects because this second operation was intended to perform before pseudopterygium would enlarge in size. Finally temporary AM patch was also applied over the entire cornea to cover the denuded central cornea caused by epithelium harvesting.
The excised corneal epithelium with AM was examined by light and transmission electron microscopy (JEM-1200CX, JOEL, Tokyo, Japan). The extent of hemidesmosomes was evaluated as previously described (13). Furthermore, immunohistochemistry was employed to determine the cytokeratin 3 and collagen IV (markers for healthy corneal epithelium and BM, respectively) expressions using monoclonal antibodies from ICN Biomedical, Inc. (AE5; Aurora, OH, U.S.A.) and Neomarkers, Inc. (Cat. #MS-747-S, Fremont, CA, U.S.A.), respectively.
Average age of the patients was 44.7±2.5 (ranging from 35-46) yr, and mean follow-up was 22.5±1.4 months (ranging from 18-39). Every subjects had severe corneal burn injuries (Hughes-Roper-Hall burn grade 3, 3 eyes; grade 4, 4 eyes). Amniotic membrane transplantation using limbal transplantation (first operation) was successfully completed in all cases without any acute complications. But, central corneal opacity and peripheral pseudopterygium developed within 6 months after this 1st operation in all cases. In vivo expanded corneal epithelial transplantation (2nd operation) was performed in order to restore these complications. The mean interval between the 1st and 2nd operation was 3.3 months (ranging from 1-6). All eyes healed successfully with epithelialization 3 to 7 days after the second operation and showed improved visual acuity (better than 20/60) except in case 6 (Table 1).
Ultrastructural changes occurring in the in vivo cultivated corneal epithelium on AM were analyzed after 1, 2, and 3 months by electron microscopy and immunohistochemical studies.
One month after in vivo cultivation, the expanded epithelium on AM consisted of 4, 5 cell layers of corneal epithelium and looked normal in appearance under the light microscope, but partial detachments were observed between basal cells and AM. Also, the cultivated corneal epithelium and growing BM were not stained by cytokeratin K3 and collagen IV respectively (Fig. 1).
However, on the third month, the cultivated epithelium exhibited well differentiated five to six stratified cell layers and was attached firmly to the AM. These findings were further confirmed by immunohistochemical study, as the cultivated suprabasal corneal epithelium was stained strongly for cytokeratin K3 and newly formed BM began to stain for collagen IV (Fig. 2).
In terms of ultrastructural changes with time, basal cells of in vivo cultivated corneal epithelium were differentiated from rudimental round cells with large nuclear cytoplasmic (N/C) ratio after 1 month to normal cuboidal cells at 3 months. The electron-dense structure of BM began to appear at 2 months and then became distinct continuous layers with well-formed hemidesmosomes at 3 months. The hemidesmosome density increased with time, i.e., 1.3, 2.2, and 3.4 per micrometer on average after 1, 2, 3 months of in vivo cultivation, respectively (Fig. 3).
The repair of severe ocular burns has been a long-standing challenge and has generated a substantial variety of medical techniques and surgical procedures, but with only limited success. In the past several years, therapeutic techniques for reconstructing the ocular surface have been greatly advanced by the introduction of limbal epithelial cell transplantation and amniotic membrane transplantation. Actually, this combination therapy has been successful for the treatment of acute stage corneal burns in most cases. But, this procedure cannot completely prevent late complications such as pseudopterygium, neovascularization, and persistent epithelial defect in some severe cases. Therefore, we need to remove these lesions to enhance vision, and a new corneal epithelium is needed as a graft material to restore the epithelial defect caused by removing these epithelial lesions. The issue was, how can we easily acquire a new intact corneal epithelium? So, we performed limbal transplantation and amniotic membrane transplantation as a first step, and then, as a second step, covered the epithelial defect using the 'in vivo' expanded corneal epithelium which was a by-product of the first operation.
In our previous work, we retransplanted 'in vivo' expanded corneal epithelium onto adjacent lesions after limbal transplantation and amniotic membrane transplantation and described its beneficial results. Here we performed amniotic membrane transplantation at the acute stage to control inflammation and to provide a BM for epithelial regeneration.
It is well known that AM can inhibit inflammation by regulating protease activity in the alkali burned cornea (14). AM contains several proteinase inhibitors and also suppresses inflammatory genes (i.e., iNOS and its related genes and matrix metalloproteinases-II and VI) in ex vivo keratocyte culture systems (4,15). The AM patch has proven to be a viable alternative method for treating chemical burns with minimal limbal damage (16,17). However, when the loss of limbal and stromal cells is severe with total corneal epithelial defects, amniotic membrane transplantation and limbal transplantation should be adopted as described for the first operation. This procedure involves two major strategies: limbal transplantation restores the stem cell population and the amniotic membrane transplantation restores the damaged BM and stroma.
The value of AM lies in its ability to restore an intact BM that is invariably damaged in severe cornea burn injury. Moreover, BM is known to support epithelial cell adhesion, differentiation and migration (18) and to suppress epithelial cell apoptosis (19). Thus AM basement membrane is an ideal substrate for supporting the growth of epithelial progenitor cells by prolonging their lifespan and maintaining their clonogenicity (20). Another way in which AM facilitates epithelialization is as a bandage contact lens that protects migrating epithelial cells from the windshield wiper action of the eyelids (21,22).
In the present study, we found that the in vivo expansion of the corneal epithelium on AM formed a well-differentiated multilayered corneal epithelium with adequate hemidesmosome density at 3 months. This finding justifies the second procedure where in vivo expanded corneal epithelial cells together with AM are removed and retransplanted to reconstruct the corneal surface that is still conjunctivalized to pseudopterygium and opacified by AM. The advantage of a limbal tissue in vivo culture is that it is tightly attached and well-formed limbal epithelial cells can then be harvested on AM.
This study presents successful results for the management of late onset complications in burned eyes. All of the cases except one (case 6) healed successfully with epithelialization 3 to 7 days after the second operation.
After one month of in vivo cultivation, the expanded epithelium on AM consisted of rudimentary corneal epithelium and incompletely formed hemidesmosomes. But, at 3 months the cultivated epithelium exhibited well-differentiated five to six stratified cell layers with adequate hemidesmosome density, although at 3 months, the extent of hemidesmosomes was more abundant than that of either 1 or 2 months, but yet subnormal.
Also, the BM began to stain with collagen IV, while epithelial cells were strongly positive for cytokeratin K3 after 3 months of in vivo cultivation. These findings indicate that the regenerating corneal epithelium on AM regains its structure and function over this period of in vivo cultivation. Unlike epithelium, the formation of BM and its related structure were still ongoing process at 3 months. Therefore, further observation is required to appraise the clinical significance of in vivo cultivated corneal epithelium.
On the other hand, we could find that pseudopterygiums and subsequent corneal opacities were developed again in 4 of 7 eyes throughout the long-term follow-up. This might result from gradual cell loss of transplanted corneal epithelia secondary to limbal insufficiency. This explanation is supported by the fact that all eyes involved in this study were undergone severe corneal burn graded as 3 or 4 according to Hugh-Roper-Hall classification (grade 3 or 4 are equivalent to 50% or more limbal ischemic area).
Therefore, new method such as additional limbal transplantation should be considered for preventing limbal insufficiency, or this transplantation of in vivo expanded corneal epithelium is recommended in corneal burn with mild to moderate limbal ischemia.
Also, our study is limited by its small sample size and pathologic condition for epithelial expansion. So, our next study will be designed to analyze epithelial phenotype and integrity of in vivo expanded corneal epithelium cultivated on living-related healthy cornea.
This report shows that in vivo cultured corneal epithelial cells on human AM may be useful as a graft material for reconstructing the damaged ocular surface and indicates that at least 3 months is needed. The findings of the present study could serve as a basis for deciding on the optimal time of retransplantation of in vivo expanded corneal epithelium.
Figures and Tables
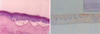 | Fig. 1First month after in vivo cultivation on amniotic membrane (AM). (A) The expanded epithelium on AM consists of 4, 5 cell layers of corneal epithelium and looks normal in appearance, though there are partial detachments (asterisk) between basal cells and AM (H&E, ×200). (B) Cultured corneal epithelium stained poorly for cytokeratin K3 (×200) and growing BM does not stain for collagen IV (inset: ×200). |
 | Fig. 2Third month after in vivo cultivation on AM. (A) The cultured epithelium exhibits well differentiated five to six stratified cell layers and is attached firmly to the AM (H&E, ×200). (B) Whole suprabasal corneal epithelium is stained strongly with cytokeratin K3 (×200) and newly formed BM is stained partially with collagen IV (arrowhead in inset: ×200). |
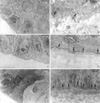 | Fig. 3EM findings of epithelial basal cells (asterisk) and hemidesmosomes (arrow) and basement membrane development. (A, B) First month (C, D) Second month (E, F) Third month. The basal cells of in vivo cultivated corneal epithelium are differentiated from rudimental round cell with large N/C ratio at the 1st month to normal cuboidal cells after 3 months of cultivation. The electron-dense structure of BM began to appear at 2 months and became distinct continuous layers with well-formed hemidesmosomes. The density of hemidesmosomes increased with time, i.e., 1.3, 2.2, and 3.4 per micrometer on average after 1, 2, 3 months of in vivo cultivation, respectively. |
Table 1
Patient profiles and surgical procedures

AMT, amniotic membrane transplantation; CLAU, conjunctival limbal autograft; TAMP, temporary amniotic membrane patch; Remov-IGSA and reT, removal of central part of in vivo expanded corneal stem cells on amniotic membrane and retransplantation to the lesion of the removed pseudopterygium; PKP, penetrating keratoplasty; ECCE+IOL, extracapsular cataract extraction and intraocular lens implantation; reT-IGSA, retransplantation of In vivo expanded corneal stem cells on amniotic membrane; OD, right eye; OS, left eye.
References
1. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986. 103:49–62.

2. Tseng SC, Chen JY, Huang AJ. Classification of conjunctival surgeries for corneal disease based on stem cell concept. Ophthalmic Clin North Am. 1990. 3:595–610.
3. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989. 96:709–723.

4. Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits protease activity on wound healing following acute corneal alkali burns. Exp Eye Res. 1998. 70:329–337.
5. Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, Prabhasawat P, John T, McLeod SD, Steuhl KP, Tseng SC. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000. 107:980–990.

6. Tsubota K, Satake Y, Ohyama M, Toda I, Takano Y, Ono M, Shinozaki N, Shimazaki J. Surgical reconstruction of the ocular surface in advanced ocular cicatricial phemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996. 122:38–52.
7. Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998. 116:431–441.

8. Park GS, Ye J, Kim JC. Stepwise surgical approach for in vivo expansion of epithelial stem cells to treating severe acute chemical burns with total limbal deficiency. Korean J Ophthalmol. 2003. 17:75–82.
9. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000. 343:86–93.

10. Koizumi N, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorder. Ophthalmology. 2001. 124:1569–1574.
11. Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK. 1965. 85:631–653.
12. Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997. 123:303–312.

13. Tisdale AS, Spurr-Michaud SJ, Rodrigues M, Hackett J, Krachmer J, Gipson IK. Development of the anchoring structures of the epithelium in rabbit and human fetal corneas. Invest Ophthalmol Vis Sci. 1988. 29:727–736.
14. Kim JC, Kim YJ, Song HJ. Down-regulation of metalloproteinases and inducible nitiric oxide synthese in keratocyte cultured with amniotic membrane extract (ARVO abstract no 1393). Invest Ophthalmol Vis Sci. 2000. 41:S265.
15. Na BK, Hwang JH, Kim JC. Analysis of human amniotic membrane components as proteinase inhibitors for development of therapeutic agent for recalcitrant keratitis. Trophoblast Res. 1999. 13:453–466.

16. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.

17. Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Ophthalmol. 1995. 9:32–46.

18. Tseng SC, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997. 124:825–835.

19. Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995. 267:891–893.

20. Meller D, Pires RT, Tseng SC. Ex-vivo preservation and expansion of human limbal epithelial progenitor cells by amniotic membrane. Invest Ophthalmol Vis Sci. 1999. 40:S329.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download