Abstract
The purpose of this study was to evaluate whether maternal serum (MS) and amniotic fluid (AF) inhibin A levels are elevated in patients who subsequently develop severe preecalmpsia, and to investigate the correlation between MS and AF inhibin A levels in the second trimester. The study included 40 patients who subsequently developed severe preecalmpsia and 80 normal pregnant women. Inhibin A levels in MS and AF were measured with enzyme-linked immunosorbent assay (ELISA). The MS and AF inhibin A levels in patients who developed severe preeclampsia were significantly higher than those in the control group (both for p<0.001). There was a positive correlation between MS and AF inhibin A levels in patients who developed severe preeclampsia (r=0.397, p=0.011), but not in the control group (r=0.185, p=0.126). The best cutoff values of MS and AF inhibin A levels for the prediction of severe preeclampsia were 427 pg/mL and 599 pg/mL, respectively; the estimated ORs that were associated with these cut-off values were 9.95 (95% CI 3.8-25.9, p<0.001) and 6.0 (95% CI 2.3-15.8, p<0.001). An elevated level of inhibin A in MS and AF at the time of second trimester amniocentesis may be a risk factor for the subsequent development of severe preeclampsia.
Preeclampsia, which affects 3% to 5% of pregnancies (1), is a pregnancy-specific disorder characterized by hypertension and proteinuria. It is among the leading causes of fetal and maternal morbidity and mortality. The etiology of the condition is unknown, but placental disorders are probably involved in the pathophysiologic mechanism (2). Therefore, a reliable and early placental marker could be extremely beneficial in detecting pregnant women at high-risk for preeclampsia.
Recently, inhibin A, a glycoprotein mainly produced by the syncytiotrophoblast of the human placenta during pregnancy has been evaluated both for the prediction of preeclampsia (3-5) as well as assessment of severity (6-8). Sebire et al. (9) reported previously that inhibin A levels might increase as early as 10 to 14 weeks of gestation, based on the observation of 7 patients who subsequently had preeclampsia. It has been shown that maternal serum levels of inhibin A are 10-fold higher in women with severe preeclampsia compared to gestational age matched controls (8). Fraser et al. (10) also reported that inhibin A levels are markedly elevated in women at the time they experience preeclampsia. In women who subsequently developed preeclampsia, inhibin A levels were found to be elevated at 13-18 weeks in a retrospective analysis of a Down's screening programme (5).
Previous study has demonstrated that inhibin A levels in maternal serum and amniotic fluid are relatively different in normal pregnant women (11). Other studies have reported amniotic fluid levels of inhibin A in chromosomally normal and Down's syndrome pregnancies (12,13). However, maternal blood and amniotic fluid obtained at the time of second trimester genetic amniocentesis are rarely used to assess the risk of preeclampsia. The aim of this study was to investigate the inhibin A level in maternal serum and amniotic fluid at the time of second trimester genetic amniocentesis in patients who subsequently develop severe preeclampsia. In addition, we compared the correlation between maternal serum and amniotic fluid inhibin A levels in both normal pregnant women and subsequently severe preeclampsia.
A case-control study was desgined with stored maternal serum and amniotic fluid obtained from women who underwent second trimester genetic amniocentesis between Octerber 2001 and December 2003 at Samsung Cheil Hospital in Seoul, Korea. The study groups consisted of pregnant women who subsequently developed severe preeclampsia and normotentive women who had a normal pregnancy outcome (term gestation with a neonate with adequate weight for gestational age).
Forty patients who developed severe preeclampsia were matched for maternal age and gestational age at sampling with 80 normotentive women who had a normal pregnancy outcome. Preeclampsia was defined as hypertension (systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg after 20 weeks' gestation) and proteinuria (≥300 mg in a 24 hr urine collection or one dipstick measurement of ≥1+) according to the Committee of Terminology of American College of Obstetricians and Gynecologists (ACOG) definition (14). Severe preeclampsia was diagnosed on the basis of diastolic blood pressure ≥110 mmHg or significant proteinuria (dipstick measurement of ≥2+) or the presence of severity evidences such as headache, visual disturbances, upper abdominal pain, oliguria, convulsion, elevated serum creatinine, thrombocytopenia, marked liver enzyme elevation, and pulmonary edema. Cases with an abnormal fetal karyotype, chromosomal abnormalities, chronic hypertension, diabetes, or renal disease at the time of amniocentesis were excluded. The control group consisted of patients who had a second trimester amniocentesis who delivered a normal neonate at term without significant medical or obstetric complications. The Ethics Committee of Samsung Cheil Hospital approved the collection of these samples and the clinical information and samples for research puroposes.
Amniotic fluid was obtained by transabdominal amniocentesis and an aliquot of amniotic fluid was centrifuged and stored at -70℃ until assay. Maternal blood was drawn at the time of second trimester genetic amniocentesis, collected into plain serum-gel tubes (Becton Dickinson, U.S.A.), centrifuged, and stored at -70℃. Inhibin A levels in amniotic fluid and maternal serum were measured with a commercially available enzyme-linked immunosorbent assay (ELISA, Diagnostic Systems Laboratories, Inc., Texas, U.S.A.) according to the manufacturer's instructions. All samples were run in duplicate. The inter- and intra-assay coefficients of variation were less than 10%.
Statistical analysis was performed using the Statistical Package for Social Sciences version 10.0 (SPSS Inc., Chicago, U.S.A.). Kolmogorov-Smirnov tests were used to test for normal distribution of the data. As inhibin A levels in this study were not normally distributed, a Mann-Whitney U tests were used for comparison of continuous variables, and proportions were compared with the χ2 and Fisher's exact tests. Spearman's rank correlation was used to assess the relationship between two variables. We used the receiver operator characteristic (ROC) analysis to determine the best cut-off value of inhibin A level for predicting severe preeclampsia and calculated the odds ratio (OR) and 95% confidence interval (CI). A p value <0.05 was considered statistical significance.
The clinical characteristics of the study population are shown in Table 1. There were statistical differences in nulliparity, gestational age at delivery, and birth weight between patients who developed severe preeclampsia and those in the control group. As expected, the blood pressures of patients who developed severe preeclampsia were significantly higher than those in the control group. In contrast, the groups were similar in terms of maternal age, indication for amniocentesis, gestational age at amniocentesis and blood sampling, and platelet count.
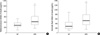
Fig. 1 displays inhibin A levels in the second trimester maternal serum and amniotic fluid. The median maternal serum and amniotic fluid levels of inhibin A were significantly higher in patients who developed severe preeclampsia than those in the control group (maternal serum: median 414 pg/mL, range 168-1,039 pg/mL vs. median 280 pg/mL, range 106-522 pg/mL, p<0.001; amniotic fluid: median 525 pg/mL, range 142-1,305 pg/mL vs. median 275 pg/mL, range 103-882 pg/mL, p<0.001). In patients who developed severe preeclampsia, there was a positive correlation between maternal serum and amniotic fluid inhibin A levels (r=0.394, p=0.011) (Fig. 2). In contrast, there was no correlation between amniotic fluid and maternal serum inhibin A levels in the control group (r=0.185, p=0.126). ROC curves were constructed to select cut-off values at which to dichotomize the level of maternal serum inhibin A or amniotic fluid inhibin A to identify the subsequent development of severe preeclampsia. The best cut-off values of each maternal serum and amniotic fluid inhibin A level for the prediction of severe preeclampsia were 427 pg/mL and 599 pg/mL; the estimated ORs that were associated with these cut-off values were 9.95 (95% CI 3.8-25.9, p<0.001) and 6.0 (95% CI 2.3-15.8, p<0.001).
We confirmed that the median second trimester maternal serum inhibin A levels in patients who subsequently developed severe preeclampsia was significantly higher than those in normal pregnant women. Our data are consistent with those of previous studies that reported an increase in serum inhibin A concentration before the onset of preeclampsia. Cuckle et al. (15) reported an increase in maternal serum inhibin A concentration at 13 to 18 weeks of gestation in 28 patients who subsequently had preeclampsia. Aquilina et al. (16) evaluated the screening efficacy of serum inhibin A determination combined with uterine artery Doppler studies at 15 to 19 weeks of gestation in 37 women who subsequently had preeclampsia. King et al. (17) reported that preeclamptic women had second trimester serum inhibin A levels 1.3-fold higher than the control mean. In contrast to these reports, some studies found no difference in second trimester serum inhibin A levels between healthy pregnant women and women who later developed preeclampsia (18,19).
In preeclampsia, there is partial or complete failure of trophoblastic invasion of the myometrial segments of the spiral arteries (2), a process that normally has taken place by 20 weeks of gestation (20). The failure of trophoblastic invasion is associated with ischaemic damage to the syncytiotrophoblast causing functional alteration of the surface layer of the syncytiotrophoblast (21). This alteration in the surface layer of the syncytiotrophoblast has been postulated as a contributory factor for the increased 'leakage' of inhibin A into the maternal circulation (22), which might explain the increase in concentration of maternal serum inhibin A in preeclampsia.
This is the first report for inhibin A levels in the second trimester amniotic fluid of the patients who subsequently developed severe preeclampsia. Our results also indicated the amniotic fluid inhibin A levels were significantly higher in patients who subsequently developed severe preeclampsia than those in normal pregnant women. Moreover, inhibin A levels in amniotic fluid were significantly higher than those in maternal serum for this disease. We speculate that increased inhibin A levels in amniotic fluid may enter maternal circulation, leading to higher detectable levels in maternal blood of patients who subsequently developed severe preeclampsia. Further investigations for the mechanism of inhibin A through the amniotic fluid to maternal blood are necessary. Our data suggest that amniotic fluid may be another significant source of inhibin A for the prediction of severe preeclampsia.
During pregnancy, the placenta produces and secretes inhibin A, which then enters both the maternal and the fetal circulation (11,12,23). The placental inhibin A that enters maternal and the fetal circulation may cause an elevation in maternal serum and amniotic fluid inhibin A levels. We observed that the amniotic fluid inhibin A levels were positively correlated with the maternal serum inhibin A levels in the patients who subsequently developed severe preeclampsia. This correlation may be associated with increased amniotic membrane permeability in patient with severe preeclampsia.
In conclusion, the second trimester maternal serum and amniotic fluid inhibin A levels in pregnant women who subsequently developed severe preeclampsia were significantly higher than those in normal pregnant women. There is a positive correlation between elevated maternal serum and amniotic fluid inhibin A levels in patients who subsequently developed severe preeclampsia. At the time of genetic amniocentesis, we suggest that the elevated level of inhibin A in maternal serum and amniotic fluid may be a risk factor for the subsequent development of severe preeclampsia, although larger studies are needed to confirm this point.
Figures and Tables
Fig. 1
Levels of maternal serum (A) and amniotic fluid inhibin A (B) in normal pregnancy (NP) and patients with severe preeclampsia (SPE). *Statistically significant, p<0.05.
References
2. Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994. 101:669–674.

3. Muttukrishna S, North RA, Morris J, Schellenberg JC, Taylor RS, Asselin J, Ledger W, Groome N, Redman CW. Serum inhibin A and activin A are elevated prior to the onset of preeclampsia. Hum Reprod. 2000. 15:1640–1645.

4. Lambert-Messerlian GM, Silver HM, Petraglia F, Luisi S, Pezzani I, Maybruck WM, Hogge WA, Hanley-Yanez K, Roberts JM, Neveux LM, Canick JA. Second-trimester levels of maternal serum human chorionic gonadotropin and inhibin 6 as predictors of preeclampsia in the third trimester of pregnancy. J Soc Gynecol Invest. 2000. 7:170–174.
5. Aquilina J, Barnett A, Thompson O, Harrington K. Second-trimester maternal serum inhibin A concentration as an early marker for preeclampsia. Am J Obstet Gynecol. 1999. 181:131–136.

6. Silver HM, Lambert-Messerlian GM, Star JA, Hogan J, Canick JA. Comparison of maternal serum total activin A and inhibin A in normal, preeclamptic, and nonproteinuric gestationally hypertensive pregnancies. Am J Obstet Gynecol. 1999. 180:1131–1137.

7. Gratacos E, Casals E, Gomez O, Aibar C, Cararach V, Alonso PL, Fortuny A. Inhibin A serum levels in proteinuric and nonproteinuric pregnancy-induced hypertension: Evidence for placental involvement in gestational hypertension? Hypertens Pregnancy. 2000. 19:315–321.
8. Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL. Activin A and inhibin A as possible endocrine markers for preeclampsia. Lancet. 1997. 349:1285–1288.

9. Sebire NJ, Roberts L, Noble P, Wallace E, Nicolaides KH. Raised maternal serum inhibin A concentration at 10 to 14 weeks of gestation is associated with pre-eclampsia. Br J Obstet Gynaecol. 2000. 107:795–797.

10. Fraser RF, McAsey ME, Coney P. Inhibin A and pro-alpha C are elevated in preeclamptic pregnancy and correlate with human chorionic gonadotropin. Am J Reprod Immunol. 1998. 40:37–42.
11. Wallace EM, Riley SC, Crossley JA, Ritoe SC, Horne A, Shade M, Ellis PM, Aitken DA, Groome NP. Dimeric inhibins in amniotic fluid, maternal serum, and fetal serum in human pregnancy. J Clin Endocrinol Metab. 1997. 82:218–222.

12. Wallace EM, Crossley JA, Groome NP, Aitken DA. Amniotic fluid inhibin A in chromosomally normal and Down's syndrome pregnancies. J Endocrinol. 1997. 152:109–112.
13. Wallace EM, D'Antona D, Shearing C, Evans LW, Thirunavukarasu P, Ashby JP, Shade M, Groome NP. Amniotic fluid levels of dimeric inhibins, pro-alpha C inhibin, activin A and follistatin in Down's syndrome. Clin Endocrinol. 1999. 50:669–673.
14. Cuningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD. Williams Obstetrics. 2001. 21st Ed. McGraw-Hill;568–569.
15. Cuckle H, Sehmi I, Jones R. Maternal serum inhibin A can predict pre-eclampsia. Br J Obstet Gynaecol. 1998. 105:1101–1103.

16. Aquilina J, Thompson O, Thilaganathan B, Harrington K. Improved early prediction of pre-eclampsia by combining second-trimester maternal serum inhibin A and uterine artery Doppler. Ultrasound Obstet Gynecol. 2001. 17:477–484.
17. King IB, Williams MA, Sorensen TK, Luthy DA. Inhibin A and activin A levels in the second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 1998. 178:S115.
18. Grobman WA, Wang EY. Serum levels of activin A and inhibin A and the subsequent development of preeclampsia. Obstet Gynecol. 2000. 96:390–394.

19. D'Anna R, Baviera G, Corrado F, Leonardi I, Buemi M, Jasonni VM. Is mid-trimester maternal serum inhibin A a marker of preeclampsia or intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002. 81:540–543.
20. Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980. 1:3–19.

21. Arnholdt H, Meisel F, Fandrey K, Lohrs U. Proliferation of villous trophoblast of the human placenta in normal and abnormal pregnancies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991. 60:365–372.

22. Redman CW. Current topic: pre-eclampsia and the placenta. Placenta. 1991. 12:301–308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download