Abstract
The survival in cases with relapsed Wilms tumor is dismal. Recently, however the introduction of new therapeutic agents and experimental strategies has improved the survival. We analysed the survival of patients with relapsed Wilms tumor according to the treatment period. During the early period 1983-1993, patients who had received two drugs were treated with doxorubicin and the others were treated with cisplatin and etoposide, whereas during the late period 1994-2004, patients were treated with combinations of cyclophosphamide/etoposide and carboplatin/etoposide. During the early period, 8 of 57 experienced relapse, and 8 of 41 relapsed during the late period. Only 2 patients treated during the early period survived in complete response (CR), whereas during the late period, 5 patients remained alive in CR, and 3 of those received high-dose chemotherapy (HDC) with autologous peripheral stem cell rescue (SCR). The estimated 5 yr event-free survival rate was 37.5% in the entire study group, 50% for patients in the late period, and 25% for patients in the early period (p=0.38). The survival in patients with relapsed Wilms tumor dramatically improved during the late period and HDC with SCR was one of the effective salvage strategies.
Approximately 85% of newly diagnosed Wilms tumor patients are now cured using multimodal therapies, but patients who relapse or who have refractory disease have a poor prognosis. According to the findings of National Wilms Tumor Studies (NWTS) II and III, the three-year overall survival rate after relapse was only 30±3% (1). Pinkerton et al. (2) reported that even a low risk group with focal relapse had a survival rate lower than 50%, thus once relapsed the prognosis is very poor. Poor prognostic factors, as identified by previous NWTS, are a stage >II, an unfavorable histology, early relapse at <12 months after initial diagnosis, previous combinatorial treatment with three agents, or abdominal relapse at the site of previous abdominal radiation therapy (1). New agents, except actinomycin, vincristine, and doxorubicin, have been investigated. Ifosfamide, etoposide, and carboplatin have been demonstrated to be active as single agents by several phase II clinical trials (3-5). The response rates to these drugs as single agents in children with relapsed Wilms tumor were 50%, 42%, and 40-53%, respectively. The combined use of these agents has been examined to identify possible synergistic anti-tumor effects for the treatment of relapsed pediatric solid tumors. The combination of carboplatin and etoposide resulted in an overall response rate of 43-83% (6), and ifosfamide and etoposide resulted in 55% (7) when used in children with a variety of pediatric solid tumors. However, none of these approaches improved long-term survival to above 30%. Consequently dose-response strategies were investigated. High dose chemotherapy (HDC) with stem cell rescue (SCR) has been tried with the aim of achieving a long-term disease free state (8-11). Although a small numbers of patients (i.e., <25 patients) in each study underwent HDC with SCR, the 4 yr event-free survival rates were reported in 40-60%. However, relapsed patients were a small heterogeneous group, and it has not been established whether this approach is superior to maintenance chemotherapy with newer agents in high-risk patients.
Here, we report on our experience of treating patients with relapsed Wilms tumor over a period of 20 yr, focusing on the survivals according to the treatment period.
From January 1983 to December 2004, 98 patients were diagnosed with Wilms tumor at the Seoul National University Children's Hospital. We divided these patients into two groups according to the date of relapse. The first group included patients who relapsed between 1983 and 1993 (the "early" group), and the second group included patients who relapsed between 1994 and 2004 (the "late" group). Among the 98 patients, 16 (16.3%) experienced relapse. In the early group 8 of 57 experienced relapse and in the late group 8 of 41 relapsed.
Nephrectomy was generally performed before chemotherapy. The tumor stage and histological type were assigned according to the guidelines of the NWTS (12). Patients with stage I were treated with Reg. EE (25 weeks) or EE4A (18 weeks), stage II with K (65 weeks) or K4 (60 weeks), and stage III and IV with DD (65 weeks) or DD4A (24 weeks). Radiation therapy was administered to patients with stage III and IV, or an unfavorable histology. Computed tomography (CT) scans of chest and abdomen were checked every three months.
At the time of relapse, any evidence of disease in the lungs was assessed by chest radiography and CT scan; in the abdomen by CT scan and ultrasonographic examination; in the skeleton by roentgenogram and bone scan; and in the bone marrow by bone marrow aspiration and biopsy. The same imaging strategy was used to assess the response to second-line treatment.
In the early group (1983-1993), doxorubicin (D) was added in cases who had received two drugs (actinomycin and vincristine), and the others were treated with cisplatin and etoposide at the time of relapse. In the late group (1994-2004), patients were treated with combinations of cyclophosphamide (440 mg/m2/day, 5 days)/etoposide (100 mg/m2/day, 5 days) and carboplatin (500 mg/m2/day, 2 days)/etoposide (100 mg/m2/day, 3 days) (13). There was no uniform approach to surgery or radiation therapy at the time of relapse. Response after two cycles of second-line chemotherapy was defined as follows: no response (NR) as a less than 50% reduction in tumor size or disease progression; partial response (PR) as a ≥50% reduction in the sum of the perpendiculars of all measurable lesions; and complete response (CR) as the disappearance of all measurable disease.
Patients with chemosensitive tumors in CR or PR after second-line treatment underwent HDC and SCR. Hematopoietic stem cells were collected through a double-lumen central venous line after chemotherapy with cyclophosphamide (1,000 mg/m2/day, 3 days) and etoposide (150 mg/m2/day, 3 days). Stem cells were mobilized using recombinant human granulocyte-colony stimulating factor (rHG-CSF) at 10 µg/kg/day after day 7 of chemotherapy. The collection was performed on the day when the peripheral white blood count was >1,000/µL with the presence of monocytes by a COBE spectra (Gambro BCT, Inc., Lakewood, CO, U.S.A.). Cells were then transported to the stem cell laboratory for counting and culture; they were stored at -196℃ until reinfusion on day 0. The conditioning regimen consisted of melphalan (140 mg/m2/day on day -7, 70 mg/m2/day on day -6), etoposide (200 mg/m2/day, on day -8 through -5) and carboplatin (400 mg/m2/day, on day -8 through -5). Cells were infused on day 0 after hydration and urine alkalinization had been achieved. rHG-CSF was given subcutaneously starting on day +1 of cell infusion at a dose of 10 µg/kg/day and was continued continued until the absolute neutrophil count was >1,000/µL for 2 consecutive days. Irradiated blood products and antimicrobial therapy were instituted when indicated. For the prophylaxis of hepatic veno-occlusive disease low molecular weight heparin (Nadroparin® and lipo-prostaglandin E1 (Eglandin®) were given from day -8 for about 1 month.
The duration of overall survival was defined as the period between the date of first relapse and either the date of death (from any cause) or the date of the patient's most recent follow-up. The duration of event-free survival was defined as the period between the date of first relapse and either the date of disease progression or relapse, the date of the most recent follow-up, or the date of death. Probabilities of overall survival and event-free survival were estimated using the Kaplan-Meier method. The log rank test was used to compare proportions, and the SPSS 12.0 software was used for the statistical analysis.
The patient characteristics are listed in Table 1. Eleven boys and five girls were enrolled in our study, and the median age at diagnosis was 29 months (range, 3-119 months). Two patients had stage I, 10 had stage II, 1 had stage III, and 3 had stage IV at the time of diagnosis. The median time from diagnosis to relapse was 11 months (range 2 months-89 months). The sites of relapse were lung (n=8), abdomen (n=5), lung and abdomen (n=1), bone and liver (n=1), and abdomen, lung, and bone marrow (n=1).
During the early period (1983-1993), two patients remained alive in CR, 3 had a second relapse, 2 had progressive disease, and 1 was lost to follow-up immediately after relapse. After 1994, 8 patients relapsed. One patient with a cleft lip and palate was lost to follow-up immediately after relapse, and another patient had progressive disease and died within 1 month from relapse. Two patients had a second relapse after 6 and 8 months of second-line treatment, respectively. One patient with second relapse in the lung was cured by chemotherapy, pulmonary radiation, and tumorectomy. Another patient with relapse in the abdomen during the initial chemotherapy (Reg. K) received Reg. DD4 chemotherapy and abdominal radiation. However, pleural effusion developed and the disease progressed despite additional chemotherapy (according to CCG 4921), and this patient died 15 months after relapse. Four patients achieved continuous CR with second-line treatment and 3 of these received HDC with autologous peripheral SCR. The numbers of infused mononuclear cell were 20.9×108/kg, 5.64×108/kg, and 10.35×108/kg and those of CD34+ cells were 9.16×106/kg, 7.7×106/kg, and 17×106/kg, respectively. All three patients had grade IV neutropenia and thrombocytopenia, and needed total parenteral nutrition. Hepatic veno-occlusive disease developed in one patient and lipo-prostaglandin E1 dose incrementation (1.7 µg/kg/day) and low dose dopamine were needed to control the disease. All three patients achieved complete recovery of hematopoiesis and had no permanent sequelae. One patient with disease relapse in abdomen, lung, and bone marrow achieved CR during the second-line treatment and then received maintenance chemotherapy and radiation therapy at the site of relapse. He has remained alive without evidence of disease for 20 months. The patient outcomes are summarized in Fig. 1.
Twelve patients out of 16 (75%) had at least one poor prognostic factor. The numbers of risk factors in individual patients are listed in Table 1. The estimated 5-yr event-free survival rates were 37.5% in the entire study group, 50% in patients in the late group, and 25% in patients in the early group (p=0.38). The overall survival rate in the late group and early group were 62.5% and 25%, respectively (p=0.28)(Fig. 2).
Suh et al. (14) have reported on the epidemiology and clinical outcomes of childhood Wilms tumor at 26 major Korean centers over the past 10 yr. The relapse rate was found to be 12% (29/246), and the estimated survival for relapsed Wilms tumor was 40.9%. Choi et al. (15) studied the prognostic factors and the efficacies of chemotherapeutic agents after relapse. They used a combination of cisplatin, etoposide, cyclophosphamide, and ifosfamide, and concluded that no specific chemotherapeutic agents significantly improved the survival of relapsed patients. Our data at the Seoul National University Children's Hospital indicate that progress has been made in the treatment of patients with relapsed Wilms tumor. Patients treated for recurrence during or since 1994 showed significantly better event-free and overall survivals than those treated earlier. This improvement in survival might be attributed to higher salvage rate after relapse. In the late group 4 of 6 remained alive in CCR whereas 2 of 5 in the early group.
Moreover, 2 patients who survived in the early cohort had no risk factors, while all patients had more than one risk factor in the late cohort. All of three patients who experienced a second relapse (RL2) in the early cohort were lost to follow-up, but one patient in the late cohort was salvaged with CCG 4921 chemotherapy, local radiation, and tumorectomy. Patients who relapsed in the late cohort received agents that were not included in their previous treatment regimens. These patients received a combination of cyclophosphamide, etoposide, and carboplatin, all of which were known to have a significant activity against Wilms tumor. Six of them achieved CR. Although these agents could achieve CR or PR (CR+PR rate; 60-80%) their long-term survival estimates were reported to be <40% (16). Tannous et al. (13) reported the results of an intergroup CCG and POG trial of a retrieval study in children with a relapsed Wilms tumor. CR (42%) and PR (36%) were achieved after two cycles of induction chemotherapy. Patients in CR were treated with cycles of maintenance chemotherapy. The reported event-free and overall survivals of these patients were 59% and 64%, respectively. Abu-Ghosh et al. (17) reported similar results for ICE chemotherapy. Ifosfamide was administered to patients with high-risk Wilms tumor at a dose of 1,800 mg/m2/day for 5 days, carboplatin 400 mg/m2/day for 2 days, and etoposide 100 mg/m2/day for 5 days. Patients received a median of four cycles of ICE chemotherapy (range 1-12), and the response to ICE was evaluated after two or more cycles. In 11 patients enrolled the response rate was 82% (CR+PR, 27%+55%), and their long-term 3-yr EFS was 63.6%. The post ICE consolidative approach was individualized and included; no more treatment, chemotherapy only, chemotherapy and radiation therapy, and surgery only. Two of 11 patients in PR received SCR.
Many groups have investigated the dose-dependent efficacies of chemotherapies in terms of prolonging the disease-free period. Our data included three patients who underwent HDC and SCR, and all of them have remained alive without any evidence of disease for 76, 53, and 31 months after relapse, respectively. Moreover, two of the three had an unfavorable histology at diagnosis and relapsed within 6 months. Pein et al. (9) of the French Society of Pediatric Oncology initiated a prospective study of HDC and SCR in 29 children with high-risk factors. The conditioning regimen used consisted of a combination of melphalan, etoposide, and carboplatin. The estimated 3 yr overall and disease-free survivals were 60% and 50%, respectively. However, late relapse has been documented at 26, 30, 47, and 53 months post HD chemotherapy. Kremens et al. (10) of the German Society of Pediatric Oncology and Hematology attempted the same strategy in 23 patients with high-risk criteria. The same conditioning regimen including melphalan, etoposide, and carboplatin was used in 20 patients. Among 23 patients relapse or progression occurred in 12 patients, and the overall and disease-free survivals were 60.9% and 48.2%, respectively, after a median follow-up of 58 months (range, 37-116 months). Toxic side effects mainly involved hematopoiesis and gastrointestinal mucosa, and the morbidity was considerable, but no treatment-related mortality occurred. All patients experienced grade IV neutropenia and fever but the rate of culture-proven infection was low. Grade III or IV gastrointestinal mucositis, nausea, and vomiting were frequently observed in over half of the patients, who needed parenteral nutritional support. Other toxicities involving liver, lung, and kidney were reversible. Campbell et al. (11) recently reported on the efficacy of a thiotepa and cyclophosphamide-based conditioning regimen. Thirteen children with relapsed Wilms tumor were enrolled, and 4 of those underwent two cycles of HD chemotherapy and SCR. After a median follow-up of 25 months, the estimated 4-yr OS and EFS rates were 73% and 60%, respectively. No increase in toxicity was observed in patients who underwent two versus one cycle of HDC and SCR. Thus for patients unable to achieve CR on standard-dose chemotherapy, tandem cycles of HDC with SCR may confer a survival advantage.
It remains to be determined whether intensive maintenance chemotherapy versus HDC with SCR is required to extend disease free survival in high-risk patients. However, HDC with SCR should be considered for patients with PR after second-line chemotherapy. Furthermore, the search for new active agents should continue to treat those with measurable disease after second line chemotherapy.
Figures and Tables
Fig. 1
Outcome according to the date of relapse. Actinomycin, vincristine and doxorubicin were used pre-1994, and subsequently carboplatin, etoposide and cyclophosphamide were used. From 1994, 4 patients were given high-dose chemotherapy and peripheral stem cell transplantation. All remain alive without event. Two pre-1994 patients and 5 post-1993 patients remain alive, respectively.
RL, relapse; CR, complete response; CCR, continuous complete response; RL2, second relapse; NR, no response; AL, alive; Exp, expired.
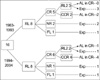
Fig. 2
Overall survival (OS, A) and event-free survival (EFS, B) as determined using the Kaplan-Meier method. Estimates of OS were 62.5% and 25% (p=0.38), and EFS were 50% and 25% (p=0.28), respectively. The above figures show a markedly improved survival rate post-1993, albeit without a statistical significance.

Table 1
Clinical and laboratory characteristics in patients with relapsed Wiilms tumor

*Risk factors included; an initial stage over 2, unfavorable histology, initial remission lasting <12 months, relapse at the irradiated site, abdomen or lung, and prior three drug chemotherapy.
RT, radiation therapy; RL, relapse; F, favorable; U, unfavorable; C, cisplatin; E, etoposide; 4921, CCG 4921; PBSCT, peripheral blood stem cell transplantation; ND, not done, A, abdomen; Lg, lung; Lv, liver; Bo, bone; BM, bone marrow; +, indicate patients alive; CCR, continuous complete response; NR, no response; RL2, second relapse; FL, follow up loss.
References
1. Grundy P, Breslow N, Green DM, Sharples K, Evans A, D'Angio GJ. Prognostic factors for children with recurrent Wilms' tumor: results from the Second and Third National Wilms' Tumor Study. J Clin Oncol. 1989. 7:638–647.

2. Pinkerton CR, Groot-Loonen JJ, Morris-Jones PH, Pritchard J. Response rates in relapsed Wilms tumor. A need for new effective agents. Cancer. 1991. 67:567–571.

3. Tournade MF, Lemerle J, Brunat-Mentigny M, Bachelot C, Roche H, Taboureau O, Olive D, Lejars O, Boilletot A, Demeocq F. Ifosfamide is an active drug in Wilms tumor: a phase II study conducted by the French Society of Pediatric Oncology. J Clin Oncol. 1988. 6:793–796.

4. Pein F, Pinkerton R, Tournade MF, Brunat-Mentigny M, Levitt G, Margueritte G, Rubie H, Sommelet D, Thyss A, Zucker JM. Etoposide in relapsed or refractory Wilms tumor: A phase II study by the French Society of Pediatric Oncology and the United Kingdom Children's Cancer Study Group. J Clin Oncol. 1993. 11:1478–1481.

5. Ettinger LJ, Gaynon PS, Krailo MD, Ru N, Baum ES, Siegel SE, Hammond GD. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Children's Cancer Group. Cancer. 1994. 15:1297–1301.

6. Pein F, Tournade MF, Zucker JM. Etoposide and carboplatin: a highly effective combination in relapsed or refractory Wilms' tumor-A phase II study by the French Society of Pediatric Oncology. J Clin Oncol. 1994. 12:931–936.

7. Kung FH, Pratt CB, Vega RA, Jaffe N, Strother D, Schwenn M, Nitschke R, Homans AC, Holbrook CT, Golembe B. Etoposide/Ifosfamide combination in the treatment of recurrent malignant solid tumors of childhood: A Pediatric Oncology Group Phase I/II study. Cancer. 1993. 71:1898–1903.
8. Garaventa A, Hartmann O, Bernard JL, Zucker JM, Pardo N, Castel V, Dallorso S, Adelbost Z, Ladenstein R, Chauvin F, Phillip T. Autologous bone marrow transplantation for pediatric Wilms' tumor: the experience of the European Bone Marrow Transplantation Solid Tumor Registry. Med Pediatr Oncol. 1994. 22:11–14.

9. Pein F, Michon J, Valteau-Couanet D, Quintana E, Frappaz D, Vannier JP, Philip T, Bergeron C, Baranzelli MC, Thyss A, Stephan JL, Boutard P, Gentet JC, Zucker JM, Tournade MF, Hartmann O. High dose melphalan, etoposide, and carboplatin followed by autologous stem cell rescue in pediatric high risk recurrent Wilms' tumor: a French Society of Pediatric Oncology study. J Clin Oncol. 1998. 16:3295–3301.
10. Kremens B, Gruhn B, Klingebiel T, Hasan C, Laws HJ, Koscielniak E, Hero B, Selle B, Niemeyer C, Finckenstein FG, Schulz A, Wawer A, Zintl F, Graf N. High-dose chemotherapy with autologous stem cell rescue in children with nephroblastoma. Bone Marrow Transplant. 2002. 30:893–898.

11. Campbell AD, Cohn SL, Reynolds M, Seshadri R, Morgan E, Geissler G, Rademaker A, Marymount M, Kalapurakal J, Haut PR, Duerst R, Kletzel M. Treatment of relapsed Wilms tumor with high-dose therapy and autologous hematopoietic stem-cell rescue: the experience at Children's Memorial Hospital. J Clin Oncol. 2004. 15:2885–2890.

12. D'Angio GJ, Evans AE, Breslow N, Beckwith B, Bishop H, Feigl P, Goodwin W, Leape LL, Sinks LF, Sutow W, Tefft M, Wolff J. The treatment of Wilms' tumor: Result of National Wilms' Tumor Study. Cancer. 1976. 38:633–646.
13. Tannous R, Giller R, Holmes E. Intensive therapy for high-risk relapsed Wilms' tumor. A CCG-4921/POG-9445 study report. Proc Am Soc Clin Oncol. 2000. 19:(Abstr 2315).
14. Suh WS, Kang IJ, Koo HH, Kook H, Kim SK, Kim HK, Kim HM, Kim HS, Park KD, Park KB, Park SK, Park JS, Park JE, Park HJ, Seo JJ, Sung KW, Shin HY, Ahn HS, Yang CH, Yoo KH, Yoo ES, Lyu CJ, Lee KC, Lee KS, Lee SY, Lee YH, Lim YT, Jang PS, Chung NG, Jeong DC, Jung HL, Cho DW, Cho B, Choi YM, Hah JO, Hwang PH, Hwang TJ. Epidemiology and clinical outcomes of childhood Wilms tumor in Korea. Korea J Pediatr Hematol-Oncol. 2004. 11:164–170.
15. Choi YD, Han SW, Choi SK, Ko WJ, Lee JS, Lee SJ, Han SJ, Hwang EH, Yu CJ, Kim BS. Prognostic factors and survival rates of preoperative and recurred cases chemotherapy in Wilms' tumor. Korean J Urol. 2000. 41:741–746.
16. Kung FH, Desai SJ, Dickerman JD, Goorin AM, Harris MB, Inoue S, Krischer JP, Murphy SB, Pratt CB, Toledano S, Wiley JM, Yu AL. Ifosfamide, carboplatin, etoposide (ICE) for recurrent malignant solid tumors of childhood: a Pediatric Oncology Group Phase I/II study. J Pediatr Hematol Oncol. 1995. 17:265–269.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download