Abstract
Heme oxygenase-1 (HO-1) has been described as an inducible protein that is capable of cytoprotection via radical scavenging and the prevention of apoptosis. Chronic exposure to hyperglycemia can lead to cellular dysfunction that may become irreversible over time, and this process has been termed glucose toxicity. Yet little is known about the relation between glucose toxicity and HO-1 in the islets. The purposes of the present study were to determine whether prolonged exposure of pancreatic islets to a supraphysiologic glucose concentration disrupts the intracellular balance between reactive oxygen species (ROS) and HO-1, and so this causes defective insulin secretion; we also wanted to evaluate a protective role for HO-1 in pancreatic islets against high glucose levels. The intracellular peroxide levels of the pancreatic islets (INS-1 cell, rat islet) were increased in the high glucose media (30 mM glucose or 50 mM ribose). The HO-1 expression was induced in the INS-1 cells by the high glucose levels. Both the HO-1 expression and glucose stimulated insulin secretion (GSIS) was decreased simultaneously in the islets by treatment of the HO-1 antisense. The HO-1 was upregulated in the INS-1 cells by hemin, an inducer of HO-1. And, HO-1 upregulation induced by hemin reversed the GSIS in the islets at a high glucose condition. These results suggest HO-1 seems to mediate the protective response of pancreatic islets against the oxidative stress that is due to high glucose conditions.
Antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, heat shock protein and heme oxygenase-1 (HO-1) are induced by various stimuli, and they represent a powerful endogenous protective mechanism that act within the pancreatic islets against free radicals (1,2). HO-1 catalyzes the rate-limiting step in heme catabolism and it generates CO and bilirubin, and bilirubin has been demonstrated as a potent antioxidant (3). In addition, HO-1 has been described as an inducible protein that is capable of cytoprotection via radical scavenging and preventing apopotosis.
Glucose toxicity is defined as nonphysiological and potentially irreversible cellular damage that results in defective insulin gene expression, and this is caused by chronic exposure to supraphysiologic glucose concentrations (4-9). The beta cell in type 2 diabetes is also adversely affected by chronic hyperglycemia and, in this sense, is also a target for secondary complications. As hyperglycemia worsens, the beta cell steadily undergoes deterioration, secretes less and less insulin, and becomes a participant in a downward spiral of loss of function (9). Recently, the overexpression of antioxidant gene products has been induced in the islets, and along with using antioxidant drugs, this helps to protect against oxidative stress (10-14). However, the relation between glucose toxicity and HO-1 in the islets is still not known completely.
Thus, we would like to determine whether prolonged exposure of the pancreatic islets to a supraphysiologic glucose concentration disrupts the intracellular balance between reactive oxygen species (ROS) and HO-1, and if this causes defective insulin secretion; we also wanted to evaluate a protective role for HO-1 against high glucose concentrations in the pancreatic islets.
The INS-1 cells (15) were grown in 5% CO2-95% air at 37℃ in RPMI-1640 medium containing 11.1 mM pyruvate, 10 mM HEPES, 50 µM 2-mercaptoethanol, 100 U penicillin/mL and 100 µg streptomycin/mL. The RPMI-1640 medium used in all the experiments contained the supplements described above. The cells were passaged weekly after they has been detached with trypsin-EDTA. All the studies were performed on INS-1 cells that were between passages 21 and 29.
The pancreata from male Wistar rats were infused with 10 mL of 1.5 mg/mL collagenase type XI (Sigma, St. Louis, MO, U.S.A.)/1% fetal bovine serum/2 units/mL RQ1 DNase (Promega, U.S.A.) solution in Medium 199 (Sigma). After it was surgically excised, the pancreas was incubated in the collagenase solution at 37℃. The undigested tissue was removed by using a 500 µm screen, and the recovered tissue was washed twice with ice-cold Hanks' balanced salt solution containing 0.1% bovine serum albumin; this was followed by centrifugation at 250×g for 4 min. The islets in the pellet were separated by using a Histopaque-1077 gradient (Sigma), and then islets were hand-picked and cultured in RPMI medium 1640 containing 10% fetal bovine serum, 11.1 mM glucose and penicillin/streptomycin/amphotericin B before the experimentation.
Hemin (Sigma) was dissolved in 0.1N NaOH and it was diluted 1:1 in phophate-buffered saline (PBS); the pH was adjusted to 7.4 and the solution was sterilized by filtration. The INS-1 cell or islets were incubated at 37℃ for 24 hr with the selected concentration of hemin.
The intracellular peroxide levels (16) were detected by flow cytometric analysis with using an oxidation-sensitive fluorescein-labeled dye, carboxylated dichlorodi-hydrofluorescein diacetate (carboxy-H2DCFDA, Molecular Probes, Carlsbad, CA, U.S.A.). Upon oxidation by intracellular ROS, the non-fluorescent dye is converted into its fluorescent form. The islets and INS-1 cells were labeled with 100 µM carboxy-H2DCFDA for 1 hr at 37℃. Following the cell loading of the dye, the islets were washed twice with PBS and then put back into culture conditions for 2 hr. The islets and INS-1 cells were then harvested, washed twice with PBS and resuspended in trypsin-EDTA (0.25% trypsin, 2 mM Na4-EDTA, Invitrogen) for 5 min at 37℃. To disperse the islets into a single cell suspension, the islets and INS-1 cells were gently passed 20 times in and out of a 200-1,000-µL tip. The cells were then washed twice with ice-cold PBS. The pellet was then resuspended in ice-cold PBS, and 2 µg/mL propidium iodide was added. The cells were analyzed using a 488 nm argon laser EPICS XL-MCL flow cytometer that was controlled by EXPO 32-ADC software (Beckman Coulter, Fullerton, CA, U.S.A.). The ROS values were analyzed using a histogram plot of carboxy-H2DCFDA (the log of the fluorescence). The results were calculated as the fold difference from the untreated control cells.
Static incubation of the islets in Krebs-Ringer buffer that contained either non-stimulatory or stimulatory concentrations of glucose was performed for 1 hr (4). The insulin levels in the Krebs-Ringer buffer samples collected from the static incubations (5.6 mM and 30 mM glucose conditions) from the islets and INS-1 cells by using a 95.5 ethanol:hydrochloric acid solution were measured by using a sensitive rat insulin radioimmunoassay kit (Linco Research Immunoassay, St. Charles, MO, U.S.A.).
The HO activity was assayed by preparing a cell homogenate from the INS-1 cells that were treated with hemin. The cell homogenate was incubated with 50 µM heme, 2 mg/mL rat liver cytosol (the source of the biliverdin reductase), 1 mM MgCl2, 3 U of glucose 6-phosphate dehydrogenase, 1 mM glucose 6-phosphate, and 2 mM NADP+ in 0.5 mL of 0.1 M potassium phosphate buffer, (pH 7.4) for 30 min at 37℃. Placing the tubes on ice terminated the reaction, and the bilirubin was extracted with chloroform as described by Wagener et al. (2). The amount of bilirubin generated was determined by using a scanning spectrophotometer (Lambda 17 UV/VIS, Perkin-Elmer Cetus Instruments, Norwalk, CT, U.S.A.) and this was defined as the difference between the wavelengths 464 and 530 nm (the extinction coefficient for bilirubin was 40/mM/cm). The HO activity was expressed as the picomoles of bilirubin formed per milligram of INS-1 cell protein per hour.
For inhibition of HO-1, pretreated HO-1 antisense ODNs (2.5 mg/mL) were transfected using a lipofection reagent to the INS-1 cells (35). The INS-1 cells at 50% confluence were washed three times with PBS and then they were cultured in medium containing 10% Nu-serum, which lacks nuclease activity (Collaborative Research Inc., Waltham, MA, U.S.A.), for 5 hr before the addition of the ODNs. N-[-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP; Boehringer Manngeim Diagnostics, Indianapolis, IN, U.S.A.) was used as a vehicle for transfecting the cells with the ODNs. The proportions used were 2 µg ODN/1 µg DOTAP/mL of medium and the protocol used was described by the manufacturer. The phosphorothioated oligonucleotides derived from the rat HO-1 sequence were synthesized at the Life technologies, U.S.A.. The sense/antisense ODNs for the HO-1 were directed against the flanking translation initiation codon in the human HO-1 cDNA. The antisense sequence for the HO-1 was 5'-GCGCTCCATCGCGGG-3', whereas the sense sequence for the HO-1 was 5'-CCCGCGATGGAGCGC-3' and for HO-1 scramble, it was 5'-GGCCCTCTACGGGCG-3'. Each ODN was phospho-rothioated on the first three bases on the 3' end and then it was purified by HPLC. The cells were incubated for 24 hr with the ODNs, and then the medium was replaced with fresh medium containing 10% fetal bovine serum; the cells were then incubated for 24 hr in the absence or presence of heme.
The pancreatic islets and the INS-1 cells are washed once in ice-cold PBS buffer. The cells were lysed in buffer (140 mM NaCL, 10 mM Tris pH 7.4, 1 mM CaCl2, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40 and 1 × complete protease inhibitors [Roche, Indianapolis, ID, U.S.A.]) and then homogenized by sonication for 2 to 20 sec on ice. The cell homogenates are centrifuged at 12,000 rpm at 4℃ for 10 min to pellet the insoluble material. The supernatant is used for Western blot analyses. The protein was determined by the bicinchonic acid (BCA) assay (Pierce, Rockford, IL, U.S.A.). The protein was fractionated by SDS-polyacrylamide gel electrophoresis and then it was electroblotted to a nitrocellulose membrane. Nonspecific binding sites are blocked by nonfat dry milk for 1 hr at room temperature. The blots are then incubated with the specific primary antibodies against HO-1 at a dilution of 1:2,000-1:5,000 for 2 hr at room temperature; this was followed by a 1 hr incubation period with the peroxidase-labeled secondary antibody at a dilution of 1:10,000. The protein bands are visualized by chemiluminescence with using the NEN detection system.
The INS-1 cells cultured for 3 days in glucose concentrations ranging from 5.6 to 30 mM had progressively greater peroxide levels with the higher glucose concentrations (Fig. 1A, p<0.05). The rat islets cultured for 3 days in 30 mM or 50 mM ribose had greater peroxide levels than that in the rat islets cultured in 11.1 mM glucose (Fig. 2B, p<0.05). In addition, the cells at higher glucose or ribose concentrations displayed a decreased GSIS (p<0.05).
The INS-1 cells were cultured for 3 days in 5.6 mM or 30 mM glucose concentrations. Compared with 5.6 mM glucose, 30 mM glucose caused an increase of the HO-1 expression and activity in the INS-1 cells (Fig.2, p<0.05).
After 3 days culture (5 hrs exposure of the ODN) of the INS-1 cells at 5.6 mM or 30 mM glucose concentrations, the intracellular peroxide level, the HO-1 expression and the GSIS were measured. HO-1 and GSIS were decreased simultaneously by treatment of the HO-1 antisense (Fig. 3, p<0.05), suggesting GSIS is associated with HO-1.
The INS-1 cells cultured for 3 days (with 1day pre-exposure of the hemin) in hemin concentrations ranging from 0.1 mM to 10 mM had progressively greater HO-1 levels with the higher hemin concentrations, and the cells had progressively smaller peroxide levels with the higher hemin concentrations (Fig. 4A, p<0.05). Similar results were also obtained in the rat islets (Fig. 4B, p<0.05).
The GSIS in both the INS-1 cells and rat islets after 3 days subculture with high glucose concentration was increased in a dose-dependent manner by additional treatment of hemin for 1 day, which was associated with HO-1 upregulation induced by hemin (Fig. 5, p<0.05).
Glucose toxicity is defined as the nonphysiological and potentially irreversible cellular damage that results in defective insulin gene expression, and this is caused by chronic exposure to supraphysiologic glucose concentrations (4-9). With using HIT-T15 cells, Robertson et al. (4) observed that cells chronically cultured for 6 months in media containing 11.1 mM glucose, a concentration exceeding what is necessary to elicit maximal insulin responses, caused a marked loss of insulin mRNA, greatly diminished levels of the insulin content and almost a complete disappearance of insulin secretion. In contrast, the HIT-T15 cells of the same passage that were serially cultured in media containing 0.8 mM glucose for 6 months retained their insulin mRNA, insulin content and their glucose-induced insulin secretion (4). The concept of glucose autoxidation along with the consequent excess generation of ROS in relation to diabetes mellitus has been proposed as early as 1987 by Wolff and Dean (17). Hunt et al. (18) demonstrated that glucose autoxidation produces hydroxyl radicals and that the hydroxyl radical scavengers protected against the glucose-induced fragmentation of protein. Earlier work by Grankvist et al. (19) demonstrated that the pancreatic islets contained relatively small amounts of antioxidant enzymes such as Cu, Zn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase. Oliveira et al. (20) reported that superoxide dismutase activity increases with the increasing glucose concentration. These observations set the stage for the increased risk for ROS-induced damage. We also found that the intracellular peroxide level was increased by a chronic exposure of high glucose conditions in both the INS-1 cells as well as rat islets, which was accompanying with a decreased GSIS (Fig. 1).
The heme oxygenases play critical roles in physiological iron homeostasis, antioxidant defense, and, as has been shown from the accumulating evidences, in the signaling pathways that employ CO as a messenger (21). Three mammalian isoforms of HO have been identified: HO-1, an inducible enzyme that is most highly concentrated in the tissues that are heavily involved in the catabolism of heme proteins (22); HO-2, a non-inducible isoform that is present at the highest concentrations in the brain and testes, and it is thought to be particularly involved in signaling pathways (23); and HO-3, an isoform with low catalytic activity and an uncertain physiological role (24). HO uses dioxygen and nicotinamide- adenine dinucleotide phosphate as cofactors, and the resulting products of the reaction are carbon monoxide, iron and biliverdin (21). Biliverdin is converted to bilirubin by a ubiquitous cytosolic enzyme biliverdin reductase (22). Both HO-2 and an inducible HO-1 have been identified in rat pancreatic islets (23-25), as well as in other tissues (26). Pancreatic islets respond to stress through the induction and activation of several stress-activated proteins. Interleukin-1β (IL-1β) induces an inflammatory response in pancreatic islets that is characterized by increased levels of inducible nitric oxide synthase (iNOS) and increased nitric oxide (NO)/nitrite levels (27-30). IL-1β and heat shock protein increase the expression of hsp70 (31,32), as well as HO-1 (23,33). The protective effect of heat shock protein (HSP) on the islet cells may be associated with the reduced lysis from NO, reactive oxygen intermediates and streptozotocin (32); but the response is nonspecific because many HSPs respond to these stimuli. On the other hand, liposomal delivery of hsp70 into islet cells protected the cells from the Il-1β effects on insulin secretion (34), suggesting that heightened levels of specific HSP can protect β cells from the inhibitory effects of the cytokine. Hemin induces the synthesis of HO-1 and so it partly counteracts the Il-1β induced inhibition of aconitase activity and glucose oxidation (33), and perhaps this occurs through antioxidant mechanisms. However, hemin has also been reported to increase insulin and glucagon secretion from normal rat islets (24). Ye and Laychock (1) reported that HO, which is also known as a heat shock protein (hsp 32), is an enzyme that may protect cells by reducing the heme levels that catalyze the oxygen radical reactions, and it appears to be a protective agent for pancreatic islets against interleukin-1β. Similarly, our study has shown that compared with 5.6 mM glucose, 30 mM glucose caused an induction of HO-1 expression and activity in the INS-1 cells (Fig. 2, p<0.05). The HO-1 expression increases in response to heme and to such stressors as UV radiation and oxidative stress, as well as to endotoxin, hormones and heavy metals (26). HO-1 induction may protect cells by reducing the heme levels that catalyze oxygen radical reactions and by elevating bilirubin, which has antioxidant properties (21). Bilirubin inhibits the autoxidation or peroxyl-radical-induced oxidation of the unsaturated fatty acids, and it apparently does so through its peroxyl radical-trapping antioxidant abilities (35-37). In the present study, we hypothesized that HO-1 can protect the suppression of GSIS resulted from glucose toxicity.
To evaluate our hypothesis, we measured GSIS after cultured with high glucose conditions in both HO-1 downregulated state and upregulated state. We observed that the GSIS was decreased by treatment of HO-1 antisense ODNs, which was accompanying with a downregulation of HO-1 expression (Fig. 3, p<0.05). Moreover, the GSIS was increased incompletely by hemin administration associating with the upregulation of HO-1 expression and activity (Fig. 4, 5). These results in this study supported our hypothesis. Thus, our results suggest that HO-1 seems to mediate the protective responses of the pancreatic islets against the oxidative stress that is due to high glucose conditions. Also, we suggested that HO-1 is one of the targets for preserving islets from glucose toxicity in diabetic state.
Figures and Tables
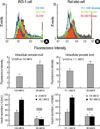 | Fig. 1The effects of high glucose on the intracellular peroxide level and Glucose stimulating insulin secretion (GSIS) in the INS-1 cells and rat islets. (A) INS-1 cells were incubated at 5.6, 22.2 or 30 mM glucose for 3 days. INS-1 cells incubated at 30 mM glucose increased levels of intracellular peroxides compared with the 5.6 mM concentration of glucose. (B) Isolated rat islets were incubated with 11.1 mM glucose or 30 mM ribose for 3 days. 30 mM ribose caused an increase of intracellular peroxide levels compared with the 11.1 mM glucose. Each cell at the high glucose or ribose concentrations showed decreased GSIS (p<0.05). Data are means±SD from 3 separate experiments. |
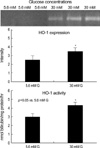 | Fig. 2The HO-1 expression and activity after 3 days subculture of the INS-1 cells. Compared with the 5.6 mM glucose concentration, 30 mM glucose caused an increase in the HO-1 expression and activity in the INS-1 cells (p<0.05). Data are means±SD from 3 separate experiments. |
 | Fig. 3The intracellular peroxide level, HO-1 expression and GSIS after 3 days culture (5 hrs exposure to the ODNs) in the INS-1 cells. HO-1 was downregulated in the INS-1 cells by the HO-1 antisense ODNs (p<0.05). Data are means±SD from 3 separate experiments. |
References
1. Ye J, Laychock SG. A protective role for heme oxygenase expression in pancreatic islets exposed to interleukin-1β. Endocrinology. 1998. 139:4155–4163.
2. Wagener FA, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of heme oxygenase isoforms on heme mediation of endothelial intracellular adhesion molecule 1 expression. J Pharmacol Exp Ther. 1999. 291:416–423.
3. Pileggi A, Molano RD, Berney T, Cattan P, Vizzardelli C, Oliver R, Fraker C, Ricordi C, Pastori RL, Bach FH, Inverardi L. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001. 50:1983–1991.

4. Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentration. J Clin Invest. 1992. 90:320–325.
5. Won KC. Oxidative stress in pancreatic islet beta-cells exposed to high glucose concentration. J Korean Diabetes Assoc. 2004. 28:250–254.
6. Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005. 280:11107–11113.

7. Sharma A, Olson LK, Robertson RP, Stein R. The reduction of insulin gene transcription in HIT-T15 beta cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcription factor expression. Mol Endocrinol. 1995. 9:1127–1134.

8. Harmon JS, Tanaka Y, Olson LK, Robertson RP. Reconstitution of glucotoxic HIT-T15 cells with somatostatin transcription factor-1 partially restores insulin promoter activity. Diabetes. 1998. 47:900–904.

9. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004. 279:42351–42354.

10. Tiedge M, Lortz S, Munday R, Lenzen S. Complementary action of antioxidant enzymes in the protection of bioengineered insulin-producing RINm5F cells against the toxicity of reactive oxygen species. Diabetes. 1998. 47:1578–1585.

11. Kralik PM, Xu B, Epstein PN. Catalase transfection decreases hydrogen peroxide toxicity in a pancreatic beta cell line. Endocr Res. 1998. 24:79–87.
12. Tiedge M, Lortz S, Munday R, Lenzen S. Protection against the cooperative toxicity of nitric oxide and oxygen free radicals by overexpression of antioxidant enzymes in bioengineered insulin-producing RINm5F cells. Diabetologia. 1999. 42:849–855.

13. Hohmeier HE, Thigpen A, Tran VV, Davis R, Newgard CB. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1β-induced cytotoxicity and reduces nitric oxide production. J Clin Invest. 1998. 101:1811–1820.
14. Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand P, Benhamou PY. Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity n human islets and INS-1 insulin-secreting cells. Diabetologia. 2000. 43:625–631.
15. Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992. 130:167–178.

16. Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci USA. 2002. 99:12363–12368.
17. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem J. 1987. 245:243–250.

18. Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J. 1988. 256:205–212.

19. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981. 199:393–398.

20. Oliveira HR, Curi R, Carpinelli AR. Glucose induces an acute increase of superoxide dismutase activity in incubated rat pancreatic islets. Am J Physiol Cell Physiol. 1999. 276:507–510.
22. George JW, Nulk K, Weiss A, Bruss ML, Cornelius CE. Biliverdin reductase activity in cattle, sheep, rabbits and rats. Int J Biochem. 1989. 21:477–481.

23. Helqvist S, Polla BS, Johannesen J, Nerup J. Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1β. Diabetologia. 1991. 34:150–156.

24. Henningsson R, Alm P, Lundquist I. Occurrence and putative hormone regulatory function of a constitutive heme oxygenase in rat pancreatic islets. Am J Physiol Cell Physiol. 1997. 273:703–709.

25. Welsh N, Margulis B, Borg LA, Wiklund HJ, Saldeen J, Flodstrom M, Mello M, Andersson A, Pipeleers DG, Hellerstrom C, Eizirid DL. Differences in expression of heat-shock proteins and antioxidant emzymes between human and rodent pancreatic islets: implications for the pathogenesis of insulin-dependent diabetes mellitus. Mol Med. 1995. 1:806–820.
26. Abraham NG, Lin JH, Schwartzman ML, Levere RD, Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1998. 20:543–558.

27. Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1β and tumor necrosis factor necrosis factor-α via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990. 276:42–44.
28. Welsh N, Eizirik DL, Bendtzen K, Sandler S. Interleukin-1β-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition in isolated of the Krebs cycle enzyme aconitase. Endocrinology. 1991. 129:3167–3173.
29. Corbett JA, Wang JL, Hughes JH, Wolf BA, Sweetland MA, Lancaster JR Jr, McDaniel ML. Nitric oxide and cyclic GMP formation induced by interleukin 1β in islets of Langerhans: evidence for a role of nitric oxide in islet dysfunction. Biochem J. 1992. 287:229–235.
30. Corbett JA, Sweetland MA, Wang JL, Lancaster JR, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA. 1993. 90:1731–1735.

31. Eizirik DL, Welsh M, Strandell E, Welsh N, Sandler S. Interleukin-1 beta depletes insulin messenger ribonucleic acid and increases the heat shock protein hsp 70 in mouse pancreatic islets without impairing the glucose metabolism. Endocrinology. 1990. 127:2290–2297.
32. Bellmann K, Wenz A, Radons J, Burkhart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995. 95:2840–2845.

33. Welsh N, Sandler S. Protective action by hemin against interleukin-1β induced inhibition of rat pancreatic islet function. Mol Cell Endocrinol. 1994. 103:109–114.

34. Margulis BA, Sandler S, Eizirik DL, Welsh N, Welsh N, Welsh M. Liposomal delivery of purified heat shock protein hsp 70 into rat pancreatic islets as protection against interleukin-1β-impaired β-cell function. Diabetes. 1991. 40:1418–1422.
35. Polte T, Abate A, Dennery PA, Achroder H. Heme oxygenase-1 is a cGMP-inducible endothelial protein and mediates the cytoprotective action of nitric oxide. Arterioscler Thromb Vasc Biol. 2000. 20:1209–1215.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download