1. Park HS. Aspirin-sensitive asthma. BioDrugs. 2000. 13:29–33.

2. Park HS, Cho YH, Kim SS, Kim HY, Nahm DH, Suh CH, Hahn MH. Prevalence of sensitivity to aspirin (ASA) and food additives in subjects diagnosed as having intrinsic asthma. J Asthma Allergy Clin Immunol. 1998. 18:662–671.
3. Delaney JC. The diagnosis of aspirin idiosyncrasy by analgesic challenge. Clin Allergy. 1976. 6:177–181.

4. Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000. 16:432–436.
5. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003. 111:913–921.

6. Jawien J. A new insight into aspirin-induced asthma. Eur J Clin Invest. 2002. 32:134–138.

7. Venter JC, Fraser CM, Harrison LC. Autoantibodies to beta2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980. 207:1361–1363.
8. Menon P, Menon V, Hilman BC, Wolf R, Bairnsfather L. Antinuclear antibodies and anticytoplasmic antibodies in bronchial asthma. J Allergy Clin Immunol. 1989. 84:937–943.

9. Szczeklik A, Nizankowska E, Serafin A, Dyczek A, Duplaga M, Musial J. Autoimmune phenomena in bronchial asthma with special reference to aspirin intolerance. Am J Respir Crit Care Med. 1995. 152:1753–1756.

10. Choi JH, Lee KW, Oh HB, Lee KJ, Suh YJ, Park CS, Park HS. HLA association in aspirin-intolerant asthma: DPB1*0301 as a strong marker in a Korean population. J Allergy Clin Immunol. 2004. 113:562–564.
11. Dekker JW, Nizankowska E, Schmitz-Schumann M, Pile K, Bochenek G, Dyczek A, Cookson WO, Szczeklik A. Aspirin-induced asthma and HLA-DRB1 and HLA-DPB1 genotypes. Clin Exp Allergy. 1997. 27:574–577.

12. Nahm DH, Lee YE, Yim EJ, Park HS, Yim HE, Kan Y, Kim JK. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002. 165:1536–1539.

13. Micheli L, Cerretani D, Fiaschi AI, Giorgi G, Romeo MR, Runci FM. Effect of acetaminophen on glutathione levels in rat testis and lung. Environ Health Perspect. 1994. 102:Suppl 9. 63–64.

14. Bucchier F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, Holgate ST, Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002. 27:179–185.

15. Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997. 22:379–394.
16. Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999. 187:567–572.

17. Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histology. 2002. 40:403–439.

18. Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am J Respir Crit Care Med. 2000. 162:2330–2336.
19. Choi JH, Nahm DH, Kim SH, Kim YS, Suh CH, Park HS, Ahn SW. Increased levels of IgG to cytokeratin 19 in sera of patients with toluene diisocyanate-induced asthma. Ann Allergy Asthma Immunol. 2004. 93:293–298.

20. Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, Nap M, Bjorklund V, Bjorklund P, Bjorklund B, Lane EB, Omary MB, Jornvall H, Ramaekers FC. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004. 297:11–26.

21. Hintner H, Romani N, Stanzl U, Grubauner G, Fritsck P, Lawley TJ. Phagocytosis of keratin filament aggregates following opsonization with IgG-anti-keratin filament autoantibodies. J Invest Dermatol. 1987. 88:176–182.

22. Szczeklik A. Aspirin-induced asthma as a viral disease. Clin Allergy. 1988. 18:15–20.

23. Martinet N, Bonnard L, Regnault V, Picard E, Burke L, Siat J, Grosdidier G, Martinet Y, Vignaud JM. In vivo transglutaminase type 1 expression in normal lung, preinvasive bronchial lesions, and lung cancer. Am J Respir Cell Mol Biol. 2003. 28:428–435.
24. Griffin M, Casadio R, Bergamini CM. Transglutaminases: Nature's biological glues. Biochem J. 2002. 368:377–396.

25. Dobashi N, Fujita J, Murota M, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ueda R, Bandoh S, Kamei T, Nishioka M, Ishida T, Takahara J. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung. 2000. 178:171–179.

26. Blobel GA, Moll R, Franke WW, Vogt-Moykopf I. Cytokeratins in normal lung and lung carcinomas. I. Adenocarcinomas, squamous cell carcinomas and cultured cell lines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984. 45:407–429.
27. Murota M, Nishioka M, Fujita J, Dobashi N, Wu F, Ohtsuki Y, Hojo S, Takahara J, Kuriyama S. Anti-cytokeratin antibodies in sera of the patients with autoimmune hepatitis. Clin Exp Immunol. 2001. 125:291–299.

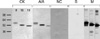
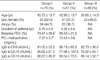
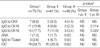




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download