Abstract
Small cell lung cancer (SCLC) is one of the most fatal cancers in humans and many factors are known to be related to its poor prognosis. Immunohistochemical (IHC) stainings were done on SCLC specimens in order to investigate the prognostic value of the apoptosis-related gene expression and the tumor proliferative maker, and the relationships among these IHC results and patients clinical characteristics, chemoresponsiveness, and survival were analyzed. The medical records of 107 patients were reviewed retrospectively. IHC stainings for p53, bcl-2 and Ki-67 expressions were performed in the 66 paraffin-embedded biopsy samples. Sixty-six out of the 107 patients were evaluable for response rate and survival. The overall response rate was 75% (95% Confidence Interval=74-76%) and the median survival time was 14 months. The median survival time of limited stage was 16 months and that of extensive stage was 10 months. The prevalence of p53, bcl-2 and Ki-67 expression was 62%, 70%, and 49%, respectively. There were no correlations among the immunoreactivities of p53, bcl-2 and Ki-67 with clinical stage, chemoresponsiveness or overall survival. The clinical stage was the only prognostic factor influencing survival. The expression rates of p53, bcl-2, and Ki-67 were relatively high in SCLC without any prognostic significance. The exact clinical role of these markers should be defined through further investigations.
Lung cancer is the most common fatal cancer worldwide and has become the leading cause of cancer deaths in Korea. The number of new cases will continue to rise (1). The risk of human cancer can be associated with environmental, occupational, and recreational exposures to carcinogens (2). Oncogenesis is related with epigenetic changes, oncogenes, tumor suppressor genes, apoptosis, and genetic changes associated with DNA repair. There have been many investigations on the prognostic role of p53, bcl-2, and Ki-67 expression in non-small cell lung cancer (NSCLC). Early reports of p53 mutation suggested a variable relationship to survival (3, 4). Another study showed that p53 mutations detectable in tumor tissues had been shown to be an independent marker for the poor prognosis in resectable stage I NSCLC (5). Data on bcl-2 expression from a study on NSCLC patients showed positive correlations with longer survival (6). There were reports on an inverse relationship between bcl-2 and p53 in NSCLC (7, 8). However, another studies did not support a relevant prognositic role for p53, bcl-2, or Ki-67 immunohistochemical markers in NSCLC regardless of stage (9, 10). No relationship was observed between the expression of Ki-67 and that of bcl-2. The relationship between a positive rate for Ki-67 and prognosis remains unclear (11). Small cell lung cancer (SCLC) has a poor prognosis. Most of the patients carry a large burden at the time of diagnosis. SCLC has been studied less than NSCLC. There was a report that bcl-2 expression did not influence survival in SCLC (12). We conducted a retrospective study on the value of mutant p53, bcl-2, and Ki-67 expressions in SCLC patients from Korea Cancer Center Hospital.
All of the patients were primarily diagnosed as SCLC at the Department of Internal Medicine of Korean Cancer Center Hospital between February 1997 and December 2002. Seventy-five of 107 SCLC patients were treated. Immunohistochemical (IHC) stainings for mutant p53, bcl-2 and Ki-67 expressions were performed in the 66 paraffin-embedded biopsy samples among the 75 member treatment group. The study group included patients (57 males and 9 females, 61 yr mean age) with cytologically or histopathologically diagnosed SCLC. Histopathological diagnoses were done by bronchoscopic biopsy, lymph node biopsy, and percutaneous lung gun biopsy. Staging procedures included physical examination, chest radiography, chest computed tomography (CT) scan, and bone scintigraphy. Brain CT/magnetic resonance image (MRI), bone marrow biopsy and other studies were optional in asymptomatic patients but mandatory in those with symptoms suggesting disseminations. Patients were staged as either limited, with disease confined to one hemithorax, or extensive, with disease beyond one hemithorax. All patients were administered one of the three chemotherapy regimens: cisplatin and etoposide (EP); cyclophosphamide, adriamycin, and vincristine (CAV); etoposide and carboplatin (EC). We established the principle that all limited disease patients would be treated by radiotherapy if the chemotherapy were effective. And then 40 patients out of all limited disease patient were treated by radiotherapy. Response categories i.e. complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were evaluated according to new response evaluation criteria in solid tumor (RECIST) guidelines (13). The CR and PR patients were considered responsive. Survival was defined as time lapse from the day of first chemotherapy course until the day of death.
Five-micrometer thickness, paraffin-embedded, tissue sections were deparaffinized in xylene and hydrated in a graded ethanol series. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. Tissue sections were heated in 10 mM sodium citrate, pH 6.0, in a microwave oven for 10 min to expose the antigens. Sections were then washed with Tris-buffered saline (TBS). The streptavidinbiotin-peroxidase complex technique (universal LSAB kit, DAKO, Glostrup, Denmark) was used for immunohistochemical stain. The sections were incubated overnight at 4℃ with the three monoclonal antibodies: p53 (1:50, DAKO, Glostrup, Denmark), bcl-2 (1:40, DAKO) and Ki-67 (1:50, Zymed, San Francisco, CA, U.S.A.). Sections were then washed with TBS and incubated with biotinylated secondary antibody for 30 min at room temperature. After washing, the sections were incubated with peroxidase-labelled streptavidin at room temperature for 30 min. Diaminobenzidine/hydrogen peroxidase was used as a chromogen and sections were counterstained with hematoxylin. When the cell nuclei were stained strongly with dark brown color, the cells were considered to be positive for p53 and Ki-67. Bcl-2 expression was noted in the cytoplasm. If more than 10% of the tumor cells were positively stained, it was considered as positive for p53 and bcl-2. In the case of Ki-67, the labeling index was determined by scanning areas with uniformly stained cells at a low magnification, followed by counting of the cells at high power field (×400). Appropriate positive and negative controls were used for all the procedures.
Median values and ranges were used to calculate patient characteristics and to compare the factors. To examine factors affecting survival, the following variables were analyzed: age, sex, disease extent, performance status, and Ki-67, p53, and bcl-2 expression. Each of these variables was divided into two categories as follows: age was divided by mean age, i.e., less than 60 yr vs. more than or equal to 60 yr; sex, male vs. female; disease extent, limited disease (LD) vs. extended disease (ED); performance status (PS), good (PS 0-1) vs. poor (PS 2-4); Ki-67, equal to or greater than 50% vs. less than 50%; p53 and bcl-2, positive vs. negative. To compare the response rate, chi-square test was used. Survival was assessed by the Kaplan-Meier method and compared with log-rank test. Hazard ratio and its 95% confidence interval for each variable were estimated by Cox proportional hazard model. A p-value lower than 0.05 was considered statistically significant. Chi-square test with Fisher exact test were used as appropriate. SPSS for Windows, 11.0 standard version, was used for all analysis.
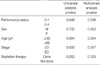
Patient characteristics are shown in Table 1. Overall response rate for evaluable 66 patients were 72%. There was no difference of the response rate among three regimens (EP, 69%; CAV, 65%; EC, 75%; p=0.83). Forty-one (62%) out of the 66 patients were positive for p53 antibodies; 25 (40%) with limited disease (LD) and 16 (24%) with extensive disease (ED) (p=0.36). The response rate for the positive p53 group was 68%, that for the negative group was 84% (p=0.15). Survival data were available for 66 patients. Median survival was 13 months for the positive p53 group, and 16 months for the negative group (p=0.50). There were no significant differences in sex, age, disease extent, performance status, and survival between patients with and without mutant p53 antibodies (Table 2). According to the analysis of prognostic factors which influenced chemotherapeutic response rate and survival, only the extent of the disease was significant for patient survival by univariate and multivariate analysis (Table 3) (Fig. 1). Median survival for the entire group was 15 months; 16 months for LD patients, and 10 months for ED patients (p=0.04). We also analyzed the survival between positive and negative p53 groups in LD patients or ED patients. In LD patients, median survival was 15 months for the positive p53 group, and 18 months for the negative group (p=0.37). In ED patients, median survival was 10 months for positive p53 and 12 months for negative (p=0.87). No correlation was found between survival and p53 expression in either LD or ED patients. Therefore p53 was not a useful prognostic indicator of SCLC.
For bcl-2, 45 (70%) out of 64 patients were positive; 26 (40%) with LD and 19 (30%) with ED (p=0.15) (Table 2). The response rate was 75% for the positive bcl-2 group, and 73% for the negative group (p=0.72). Median survival was 16 months for positive bcl-2, and 15 months for the negative group (p=0.46). In LD patients, median survival was 15 months for the positive bcl-2 group and 19 months for the negative group 19 months (p=0.37). In ED patients, median survival was 9 months for positive bcl-2, and 12 months for negative (p=0.16). No correlation was found between survival and bcl-2 expression in either LD or ED patients. Like mutant p53, there were no significant differences in sex, gender, disease extent, performance status, and survival between patients with and without bcl-2 expression.
We separately analyzed Ki-67 antigen expression both above and below 50%. Thirty-two (49%) out of 65 were in the above 50% group, 22 (34%) with LD and 10 (15%) with ED (p=0.60) (Table 2). The response rate was 69% for the above 50% group, and 79% for the below group (p=0.55). Median survival was 16 months for the above 50% group, and 15 months for the below group (p=0.21). In LD patients, median survival was 16 months for the above 50% group, and 16 months for the below group (p=0.37). In ED patients, median survival was 12 months for the above 50% group and 10 months for the below group (p=0.65). No correlation was found between survival and Ki-67 expression in either LD or ED patients. There were no significant differences in sex, gender, disease extent, performance status, and survival according to Ki-67 expression.
The p53 protein is recognized as an important cell regulatory factor that arrests the growth of cells containing damaged DNA. A reversible arrest in the G1 phase of the cell cycle enables DNA repair before DNA synthesis. When appropriate repair is not possible, p53 expression may trigger apoptosis, a reversible process culminating in cell death. If normal, wild type p53 function is lost, the treatment is relatively resistant, as a result of deficiency of p53-dependent apoptosis (14, 15). The p53 mutation is the most common genetic mutation in cancer. Thus, the mutated gene loses its natural tumor suppressor function allowing damaged cells to divide unchecked and finally to become malignant cells. There are still controversies concerning the prognosis and survival in lung cancer patients. Some studies reported that p53 mutation had been associated with poor prognosis and shorter survival in NSCLC (5, 8, 16, 17). However, some others reported no such correlation in NSCLC (3, 9, 10, 18). Others reported a favorable prognosis in NSCLC (4, 19). There have been fewer studies in SCLC than in NSCLC. Some studies showed that bcl-2 expression and p53 mutation had no relation to survival in SCLC (9, 10, 12, 20). Another study divided their SCLC patients into limited and extensive-stage disease, after which p53-antibody positivity emerged as an independent marker of poor prognosis in LD but had no relation to survival (21). One study indicated that p53 played an important role as a determinant of chemosensitivity in SCLC and that p53 immunostaining could be used in clinical practice to determine the presence of tumor-chemoresistance (22). Kenichi et al. reported that patients with expression of mutant p53 protein showed lower response rate than those having p53-negative tumors and were less sensitive to anticancer drugs (23). Our study, however, indicated that p53 antibody had no relation to survival.
The bcl-2 proto-oncogene is encoded by a 230-kb gene that gives rise to a 24- to 26-kDa protein that is localized in the inner mitochondrial membrane, and, to a lesser extent, in the cell membrane (24). The major function of bcl-2 appears to be the inhibition of apoptosis or programmed cell death, whereas bax, bad, bak, and others promote cell death (25). It is well documented that bcl-2 becomes deregulated in tumor cells as a result of translocation into the immunoglobulin heavy-chain locus, and is therefore constitutively activated in follicular lymphoma (26). In epithelial tumors, no genetic change of bcl-2 has been demonstrated, in contrast to lymphocytic neoplasia. However, bcl-2 expression has been described in a series of solid tumors, particularly in NSCLC and in breast cancer (27). An in vitro study showed that bcl-2 expression may be related to chemoresistance due to inhibition of drug-induced apoptosis (28). Thus multidrug resistance is probably linked, at least in part, to high levels of bcl-2 expression. Bcl-2 blocks the cell death pathway (apoptosis) and is not directly associated with cell proliferation (29). Studies examining the association of bcl-2 expression with survival in NSCLC have been contradictory, with some reporting bcl-2 as an indicator of better survival (6, 7), while other showed no survival differences according to bcl-2 status (30, 31). There have been few studies in SCLC. From our results, bcl-2 expression was not an independent predictor of survival in SCLC.
BrdU (bromodeoxyuridine), PCNA (proliferating cell nuclear antigen), Ki-67, and others have been used as cell proliferation markers. PCNA, a 36-kilodalton, nuclear polypeptide that is related to cell proliferation, is identical to cyclin, which is a protein that appears in the proliferative phase of cells (32). Being synthesized during the late G1 to S phase, PCNA is an auxillary protein for DNA polymerase δ. Ki-67, a more reliable proliferating marker, reacts with nuclear antigen that is present only in proliferating cells (G1, S, G2, and M phase), not in resting (G0) cells (33-35), and thereby provides a reliable method for evaluating tumor growth fraction in many malignant tumors, including lung cancer (36). It appears to be a useful prognostic marker of NSCLC, especially in the early stage (37). Compared to p53 and bcl-2, the relationship between Ki-67 and survival has been studied less extensively in NSCLC, and especially so in SCLC. Our results showed that Ki-67 expression had no relation to survival in SCLC.
From our results, the apoptosis-related genes, mutant p53 and bcl-2, and the proliferative marker, Ki-67, were found to be useful in the diagnosis of SCLC patients because of their relatively high prevalence (38, 39). However, as they did not show clinical significance for prognosis or survival, further investigation will be required to confirm the clinical role of these markers.
Figures and Tables
References
1. Ministry of Health and Welfare. 2002 Annual reports of the Korea Central Cancer Center Registry (Published in 2003). 29.
2. Harris CC. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res. 1991. 51:5023S–5044S.
3. McLaren R, Kuzu I, Dunnill M, Harris A, Lane D, Gatter KC. The relationship of p53 immunostaining to survival in carcinoma of the lung. Br J Cancer. 1992. 66:735–738.

4. Lee JS, Yoon A, Kalapurakal SK, Ro JY, Lee JJ, Tu N, Hittleman WN, Hong WK. Expression of p53 oncoprotein in non-small cell lung cancer: a favorable prognostic factor. J Clin Oncol. 1995. 13:1893–1903.
5. Harpole DH, Herndon JE 2nd, Wolf WG, Iglehart JD, Marks JR. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology and oncoprotein expression. Cancer Res. 1995. 55:51–56.
6. Pezzella F, Turley H, Kuzu I, Tungerkar MF, Dunnil MS, Pierce CB, Harris A, Gatter KC, Mason DY. Bcl-2 protein in non-small cell lung carcinoma. N Engl J Med. 1993. 329:690–694.
7. Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Angeletti CA, Basolo F, Bevilacqua G. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small cell lung cancer. Br J Cancer. 1995. 71:1003–1007.
8. Ishida H, Irie K, Itoh T, Furukawa T, Tokunaga O. The prognostic significance of p53 and bcl-2 expression in lung adenocarcinoma and its correlation with Ki-67 growth fraction. Cancer. 1997. 80:1034–1045.
9. van de Vaart PJ, Belderbos J, de Jong D, Sneeuw KC, Majoor D, Bartelink H, Begg AC. DNA-adduct levels as a predictor of outcome for NSCLC patients receiving daily cisplatin and radiotherapy. Int J Cancer. 2000. 89:160–166.

10. Cagini L, Monacelli M, Giustozzi G, Moggi L, Bellezza G, Sidoni A, Bucciarelli E, Darwish S, Ludovini V, Pistola L, Gregorc V, Tonato M. Biological prognostic factors for early stage completely resected non-small cell lung cancer. J Surg Oncol. 2000. 74:53–60.

11. Tungekar MF, Gatter KC, Dunnil MS, Mason DY. Ki-67 immunostaining and survival in operable lung cancer. Histopathology. 1991. 19:545–550.

12. Maitra A, Amirkhan RH, Saboorian MH, Frawley WH, Ashfaq R. Survival in small cell lung carcinoma is independent of Bcl-2 expression. Hum Pathol. 1999. 30:712–717.

13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij G, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000. 92:205–216.

14. Kastan MB, Canman CE, Leonard CJ. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995. 14:3–15.

15. Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994. 54:4855–4878.
16. Quinlan DC, Davidson AG, Summers CL, Warden HE, Doshi HM. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992. 52:4828–4831.
17. Laudanski J, Burzykowski T, Niklinska W, Chyczewski K, Furman M, Niklinski J. Prognostic value of serum p53 antibodies in patients with resected non-small cell lung cancer. Lung Cancer. 1998. 22:191–200.

18. Mitsudomi T, Suzuki S, Yatabe Y, Nishio M, Kuwabara M, Gotoh K, Hatooka S, Shinoda M, Suyama M, Ogawa M, Takahashi T, Ariyoshi Y, Takahashi T. Clinical implications of p53 autoantibodies in the sera of patients with non-small cell lung cancer. J Natl Cancer Inst. 1998. 90:1563–1568.
19. Bergqvist M, Brattstrom D, Larsson A, Holmertz J, Hesselius P, Losenberg L, Wagenius G, Brodin O. P53 auto-antibodies in non-small cell lung cancer patients can predict increased life expectancy after radiotherapy. Anticancer Res. 1998. 18:1999–2002.
20. Rosenfeld MR, Malats N, Schramm L, Graus F, Cardenal F, Vinolas N, Rosell R, Tora M, Real FX, Posner JB, Dalmau J. Serum anti-p53 antibodies and prognosis of patients with small cell lung cancer. J Natl Cancer Inst. 1997. 89:381–385.
21. Zalcman G, Tredaniel J, Schlichtholz B, Urban T, Milleron B, Lubin R, Meignin V, Couderc LJ, Hirsch A, Soussi T. Prognostic significance of serum p53 antibodies in patients with limited stage small cell lung cancer. Int J Cancer. 2000. 89:81–86.
22. Rodriguez-Salas N, Palacios J, Moreno G, de Castro J, Gonzalez-Baron M, Gamallo C. Correlation of p53 oncoprotein expression with chemotherapy response in small cell lung carcinomas. Lung Cancer. 2001. 34:67–74.

23. Gemba K, Ueoka H, Kiura K, Tabata M, Harada M. Immunohistochemical detection of mutant p53 protein in small cell lung cancer: relationship to treatment outcome. Lung Cancer. 2000. 29:23–31.
24. de Jong D, Prins FA, Mason DY, Reed JC, van Ommen GB, Kluin PM. Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994. 54:256–260.
26. Cleary ML, Sklar J. Nucleotide sequence of a t (14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci USA. 1985. 82:7439–7443.
27. Harris AL. What does bcl-2 mean in solid tumors-friend or foe? Ann Oncol. 1994. 5:388–390.
28. Reed JC, Kitada S, Takayama S, Miyashita T. Regulation of chemoresistance by the bcl-2 oncoprotein in non-Hodgkin's lymphoma and lymphocytic leukemia cell lines. Ann Oncol. 1994. 5:Suppl 1. 61–65.

29. Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992. 80:879–886.

30. Gaffney EF, O'Neil AJ, Staunton MJ. Bcl-2 and prognosis in non-small-cell lung carcinoma. N Engl J Med. 1994. 330:1757–1758.

31. Kim YC, Park KO, Kim HJ, Choi IS, Park CS, Juhng SW. DNA ploidy and proliferative activity in bcl-2 expressed non-small cell lung cancer. Korean J Intern Med. 1996. 11:101–107.

32. Mathews MB, Bernstein RM, Franza BR Jr, Garrels JI. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984. 309:374–376.

33. Landberg G, Ross G. Proliferating cell nuclear antigen and Ki-67 antigen expression in human hematopoietic cells during growth stimulation and differentiation. Cell Prolif. 1993. 26:427–437.
34. Wilson GD, Saunders MI, Dische S, Daley FM, Robinson BM, Martindale CA, Joiner B, Richman PI. Direct comparison of bromodeoxyuridine and Ki-67 labelling indices in human tumours. Cell Prolif. 1996. 29:141–152.

35. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984. 133:1710–1715.
36. Tubiana M, Courdi A. Cell proliferation kinetics in human solid tumors: relation to probability of metastatic dissemination and long-term survival. Radiother Oncol. 1989. 15:1–18.

37. Scagliotti GV, Micela M, Gubetta L, Leonardo E, Cappia S, Borasio P, Pozzi E. Prognostic significance of Ki-67 labelling in resected non small cell lung cancer. Eur J Cancer. 1993. 29:363–365.

38. Wang DG, Johnston CF, Sloan JM, Buchanan KD. Expression of Bcl-2 in lung neuroendocrine tumors: comparison with p53. J Pathol. 1998. 184:247–251.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download