Abstract
Cerebellum is a key structure involved in motor learning and coordination. In animal models, motor skill learning increased the volume of molecular layer and the number of synapses on Purkinje cells in the cerebellar cortex. The aim of this study is to investigate whether the analogous change of cerebellar volume occurs in human population who learn specialized motor skills and practice them intensively for a long time. Magnetic resonance image (MRI)-based cerebellar volumetry was performed in basketball players and matched controls with V-works image software. Total brain volume, absolute and relative cerebellar volumes were compared between two groups. There was no significant group difference in the total brain volume, the absolute and the relative cerebellar volume. Thus we could not detect structural change in the cerebellum of this athlete group in the macroscopic level.
Cerebellum integrates the neuronal activities of diverse structures involved in performing motor actions. The cerebellum is also implicated in motor learning process, associative learning (1-4), and various cognitive or sensory discrimination tasks (5-9). Studies on the cerebellum have contributed to our understanding of brain development, maturation, and plasticity.
Neuronal plasticity in the cerebellum has been reported in several experimental paradigms with animals. Acrobatically trained rats showed greater numbers of synapses on a Purkinje cell compared to exercise or inactive animals. The volume of molecular layer was also larger in the acrobat-trained group than in the exercise or inactive group (10). Mice allowed to exercise during postnatal period had Purkinje cells with larger dendritic trees and greater numbers of dendritic spines than sedentary littermates (11).
Thanks to recent developments in medical imaging technique such as computerized tomography (CT) or magnetic resonance imaging (MRI), in vivo morphometric analyses of human brain became possible. Cerebellar size with respect to physiological variables such as age, gender, intelligence, and motor skill was studied by several investigators (12-16). The recent MRI-based cerebellar volumetric study of musicians suggested a possibility of macroscopic plasticity in the human cerebellum (17, 18). Since basketball players practice motor skills everyday for a long time, it is possible to conceive that basketball-related motor learning could affect the morphological plasticity of cerebellum. Therefore, we hypothesize that the absolute (aCV) or the relative cerebellar volumes (rCV) of basketball players are greater than that of controls.
In order to investigate macroscopic cerebellar plasticity associated with motor skill learning in humans, we compared the size of total brain volume, absolute cerebellar volume, and relative cerebellum volume of basketball players with those of control group.
19 male basketball players were recruited from several university teams in Korea through direct visiting interviews as an athletic group (AG). The athletes, whose heights over 190 cm, were ruled out in this study to minimize the variation as maximal as possible. 20 healthy control group (CG) subjects were recruited through advertisements at Korea university web pages. Among the volunteers, subjects whose age and height matched the basketball players and who would not do any regular exercise were selected for this study.
All procedures were fully explained to the subjects and the duration of exercise training was checked. Through the history of alcohol consumption and a neurological examination by a neurologist of Korea University Medical Center, any individual with a possible neurological abnormality were excluded in this study.
Body weights and heights were measured before MRI scanning. Table 1 represents average age, height, and weight of both groups. The members of AG have played basketball for 8 yr in average and they have spent plenty of day time for highly skilled basketball training.
Magnetic resonance imaging was performed on 1.5-telsa Magnetom vision (Siemens, Erlangen, Germany). The following parameters were used for the volumetric acquisition: TR=9.7 msec, TE=4 msec, flip angle=12 degree, slice thickness=continuous 2.0 mm, matrix 192×256.
After the acquisition of MR images, the DICOM (Digital Imaging and Communications in Medicine) files were transferred to an IBM compatible PC from Sun workstation with V-work software version 3.5 (CyberMed, Korea).
Using 3-D medical software package (CyberMed, Korea) used in a previous study (19), brain tissue on the MR images were separated from non-brain tissue (skull and meninges) for whole brain and cerebellar volumetric measurement. Threshold and region-growing technique were applied to generate a new image, representing brain without any scalp and skull (20, 21). Manual tracing was used to eliminate any remaining meninges. The cut-off between the brainstem and spinal cord was the last horizontal slice including cerebellum. Although this is somewhat arbitrary, there are no obvious and accepted gross anatomical landmarks to distinguish brainstem from spinal cord on MR images. Using the last horizontal slice including cerebellum, in brains that were anterior commissure (AC)-posterior commissure (PC) aligned, ensured that the cut-off was reliable (18).
The cerebellum was segmented manually from the brainstem and cerebellar peduncles according to neuroanatomical landmarks (18, 22, 23) and criteria similar to those adopted in the previous volumetric studies of the cerebellum (24, 25). On sagittal slices, the cerebellar peduncles were removed from the cerebellar white matter according to the following procedure (Fig. 1): on the mid-sagittal slice a vertical line was drawn (at the angle of 90 degree to a line connecting the anterior and posterior commissure) which touched the posterior border of the inferior colliculus. This perpendicular line was overlaid on all sagittal slices and was used as a guide to divide the cerebellar peduncles from the brainstem. Cerebellar cortex anterior to this line was not expelled and was manually traced as part of the cerebellum (note in Fig. 1 that this included parts of the anterior lobe, biventer and flocculus on more lateral slices and of the tonsils and anterior lobe of the vermis on more medial slices). The final segmented cerebellum consisted of the cerebellar hemispheres, deep nuclei and vermis (18).
Two raters measured the total brain volume (tBV), the absolute (aCV) and the relative cerebellar volumes (rCV). The inter-rater reliability (two tailed, Pearson correlation coefficient) in tBV, aCV and rCV was 0.94 (p=0.00), 0.93 (p=0.00) and 0.96 (p=0.00), respectively. The average of two raters was used for analysis.
tBV, aCV and rCV were calculated by summing data obtained by multiplying each area by slice thickness (2 mm) using 3-D model of the total brain and the absolute cerebellum (Fig. 2). We chose to normalize aCV to tBV in order to exclude the inter-subject variability in tBV as a source of variance in aCV measurements, by calculating rCV in each subject as a percent ratio of their tBV, where rCV (%)=aCV/tBV×100. There is precedence in selecting brain volume or brain weight rather than body height to normalize brain morphometric data (18, 26, 27).
Statistical analyses were performed using SPSS for Window, version 12.0 (SPSS Inc., Chicago, IL, U.S.A.). Student's t-tests were performed to assess the effect of motor learnig on tBV, aCV, and rCV. All analyses were two-tailed, and a p-value <0.05 was considered as statistically significant.
The comparison of tBV between AG and CG revealed no significant effect of persistent motor learning (Table 2; Student t-test p=0.21). The average tBV of AG was 1355.64±73.20 cm3, whereas that of CG was 1390.14±94.96 cm3 (Table 2).
The volumetry of aCV showed no significant difference between two groups (Table 2; Student t-test p=0.07). The average aCV of AG was 138.92±9.06 cm3 and that of CG was 145.56±13.06 cm3.
The aCV of both groups in this study was slightly smaller than the result of Filipek et al. (152±10.5 cm3) (24), and was larger than that of average Korean (126±10.38 cm3) which was previously reported by Rhyu et al. (14). Our result was concomitant with that of Chung et al. (141.85±12.92) (16). This implies that sampling and volumetry are reliable.
The effect of motor learning on rCV showed no statistical differences between AG and CG group (Table 2; Student t-test p=0.38). The average rCV of AG was 10.26±0.65 cm3, whereas that of CG was 10.49±0.97 cm3.
Through the morphometric analyses of cerebellum in basketball players, we could not detect any statistical differences in tBV, aCV, and rCV.
Recent morphometric analysis of Albert Einstein's brain implied that his exceptional intelligence might be associated with larger inferior parietal lobe than that of age-matched control group (28). The relatively larger cerebellum of musicians suggested that acquisition and continual practice of complex motor skill may contribute to the macroscopic plasticity (17, 18) as in other animal training models, in which the data suggested strong correlation between motor skill learning and cerebellar plasticity (1).
Although we tried to match the age and height as possible, there were significant difference between AG and CG. The previous study clearly demonstrated that cerebellar volume was comparatively stable during young adults, but considerable reductions and increasing variability became obvious around the age of 50 yr (25, 29) and there was little correlation between height and cerebellar volume (16). So, it was thought that height and age variable did not affect the result of this study.
This study failed to detect macroscopic plasticity in the cerebellum. This failure might come from different natures of the motor skills used by musicians and basketball players. Playing musical instruments consists of relatively fine movements, whereas playing basketball consists of coarser movements using big muscles. The cerebellar plasticity according to the properties of motor skills needs to be explored further.
Differential responses of neurogenesis in adult mice according to exercise modules may support this hypothesis. Mice that had access to a running wheel have the greater number of newly formed brain cells compared with the mice of the control or swimming group (30). Another example can be found in acrobatically trained rats, whose volume of cerebellar molecular layer is bigger than those of rats received forced or voluntary exercises, or raised in inactive condition (1). Unlike the animal subjects, human subjects do various kinds of daily physical activities even though they do not do regular exercises. The restriction of motor activity in the animal experiments is much more easily done compared to that in the human experiments. We might observe microscopic plasticity in the cerebellum of AG as acrobat trained rats, if we could ran ultra-structural analysis.
In conclusion, this study finds no significant differences in tBV, aCV, and rCV between basketball players and non-basketball players. Although macroscopic plasticity was not observed in this study, present results might be valuable data in that this is the first comparative study of cerebellar volume between basketball players and non-basketball players. Longitudinal, functional levels study and lobular volumetry may be required to demonstrate this hypothesis.
Figures and Tables
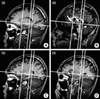 | Fig. 1Landmark-based separation of the cerebellar peduncles from the cerebellar white matter and the brainstem. On the mid-sagittal slice (slice 46/90 in this subject), perpendicular lines are drawn at a 90 degree angle to the bi-commissural line at the AC and PC (arrows) and through the posterior border of the inferior colliculus (arrowhead). These lines are superimposed on all sagittal slices and the latter perpendicular line is used to differentiate the cerebellar peduncles from the brainstem. (slice numbers are placed on top left corner of each slice). |
References
1. Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990. 87:5568–5572.

2. Kleim JA, Swain RA, Czerlanis CM, Kelly JL, Pipitone MA, Greenough WT. Learning dependent dendritic hypertrophy of cerebellar stellate cells: plasticity of local circuit neurons. Neurobiol Learn Mem. 1997. 67:29–33.
3. Mauk MD, Garcia KS, Medina JF, Steele PM. Does cerebellar LTD mediate motor learning? Toward a resolution without a smoking gun. Neuron. 1998. 20:359–362.

4. Thach WT. A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem. 1998. 70:177–188.

5. Kim SG, Ugurbil K, Strick PL. Activation of a cerebellar output nucleus during cognitive processing. Science. 1994. 265:949–951.

6. Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994. 4:8–26.

7. Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emisson tomography study of the short-term maintenance of verbal information. J Neurosci. 1996. 16:808–822.
8. Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996. 272:545–547.

9. Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997. 725:1940–1942.

10. Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998. 69:274–289.

11. Pysh JJ, Weiss GM. Exercise during development induces an increase in Purkinje cell dendritic tree size. Science. 1979. 206:230–232.

12. Paradiso S, Andreaen NC, O'Leary DS, Arndt S, Robinson RG. Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol. 1997. 10:1–8.
13. Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children-a volumetric imaging study. Brain. 1996. 119:1763–1774.
14. Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999. 270:149–152.

15. Chung SC, Choi DY, Lee BY, Lee BS, Eom JS, Sohn JH. Measuring of the cerebellar volume of normal Koreans in their 20s and 40s using magnetic resonance imaging. J Korean Radiol Soc. 2004. 51:489–493.

16. Chung SC, Lee BY, Tack GR, Lee SY, Eom JS, Sohn JH. Effects of age, gender, and weight on cerebellar volume of Korean people. Brain Res. 2005. 1042:233–235.
17. Schlaug G, Lee LH, Thangraj V, Edelman RR, Warach S. Macrostructural adapation of the cerebellum in musicians. Soc Neurosci Abstr. 1998. 24:2118. (842.7).
18. Hutchinson S, Lee LH, Gaab N, Schlaug G. Cerebellar volume of musicians. Cereb Cortex. 2003. 13:943–949.

19. Kim HJ, Yoon HR, Kim KD, Kang MK, Kwak HH, Park HD, Han SH, Park CS. Personal-Computer-based three dimensional reconstruction and simulation of maxillary sinus. Surg Radiol Anat. 2003. 24:393–399.
20. Huang Y, Knorr U, Schlaug G, Seitz RJ, Steinmetz H. Segmentation of MR images for partial-volume-effect correction and individual integration with PET images of the human brain. J Cereb Blood Flow Metab. 1993. 13:S315.
21. Peters M, Jancke L, Zilles K. Comparison of overall brain volume and midsagittal corpus callosum surface area as obtained from NMR scans and direct anatomical measures: a within-subject study on autopsy brains. Neuropsychologia. 2000. 38:1375–1381.

22. Courchesne E, Press GA, Murakami J, Berthoty D, Grafe M, Wiley CA, Hesselink JR. The cerebellum in sagittal plane-anatomic-MR correlation: 1. The vermis. AJR Am J Roentgehol. 1989. 153:829–835.

23. Press GA, Murakami J, Courchesne E, Berthoty DP, Grafe M, Wiley CA, Hesselink JR. The cerebellum in sagittal plane anatomic MR correlation: 2. The cerebellar hemispheres. AJR Am J Roentgenol. 1989. 153:837–846.
24. Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994. 4:344–360.

25. Luft AR, Skalej M, Welte D, Kolb R, Burk K, Schulz JB, Klockgether T, Voigt K. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med. 1998. 40:143–151.

26. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum: a post-mortem morphological study. Brain. 1989. 112:799–835.
27. Peters M. Sex differences in human brain size and the general meaning of differences in brain size. Can J Psychol. 1991. 45:507–522.

28. Witelson SF, Kigar DL, Harvey T. The exceptional brain of Albert Einstein. Lancet. 1999. 353:2149–2153.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download