Abstract
Regulatory T cells, which stimulate or inhibit the effector functions of distinct T cell subsets, are critical in the control of the immune response. We investigated the effect of TGF-β and IL-10 on T cell subsets according to the Th1/Th2 immune status. Sixty-two patients with asthma and 38 patients with pulmonary tuberculosis were included. Allergy skin tests, tuberculin tests, and chest radiography were performed. The levels of circulating IL-4, IFN-γ, TGF-β1, and IL-10 were measured using ELISA. The level of TGF-β1 was higher in patients with asthma than in those with tuberculosis, but the IL-10 levels were the same between the asthma and tuberculosis groups. Atopy was unrelated to the tuberculin response. The IFN-γ level was correlated with the IL-10 level, and the level of IL-4 was unrelated to the IL-10 or TGF-β1 level. The level of IL-10 was higher in the negative tuberculin reactors than in the positive tuberculin reactors among patients with asthma, and TGF-β1 was higher in the positive tuberculin reactors than in the negative tuberculin reactors among patients with tuberculosis. These results demonstrate that the regulatory effects of circulating TGF-β and IL-10 on T cell cytokines may be different between Th2-type asthma and Th1 tuberculosis.
Evidence is accumulating that CD4+ lymphocytes with a T-helper 2 (Th2) cytokine pattern play an important role in the pathogenesis of asthma (1). These cells orchestrate the recruitment and activation of the primary effector cells of the allergic response (mast cells and eosinophils), through the release of cytokines such as interleukin (IL)-4, IL-5, and IL-13. CD4+ T cells secrete a characteristic series of lymphokines, including IL-2, which is a growth factor that stimulates the clonal expansion of T cells, and interferon (IFN)-γ, which is an important mediator of macrophage activation during Mycobacterium tuberculosis infection (2). The balance between the two T-cell subsets is important for allergic sensitization. T-helper 1 (Th1) cells activate mononuclear phagocytes that are involved in cell-mediated immunity, while Th2 cells dominate asthma and allergic diseases (3).
Shirakawa et al. (4) reported that a positive tuberculin response in bacillus Calmette-Guerin (BCG)-vaccinated schoolchildren in Japan correlated with a lower incidence of atopic disorders. Th1 cells secreting IFN-γ regulate Th2 cells and may be involved in down-regulating the Th2-driven airway hyperreactivity and asthma.
Regulatory CD4+ T cells (Tr) appear to control the development of asthma and allergy. To date, four major types of Tr have been found: Th3 cells, Tr cells, CD4+CD25+ cells, and natural killer T cells (5). Either TGF-β or IL-10 has regulatory or suppressive properties that inhibit the effects of pathogenic autoreactive T cells (6). The mechanisms that protect against the development of allergic disease and asthma are not fully understood.
The authors are unaware of any data that have been published investigating Th1, Th2, and regulatory cytokines in patients with both Th2-type asthma and Th1-type active pulmonary tuberculosis. Therefore, we analyzed the tuberculin response and atopy through the Th1 and Th2 immune status in patients with asthma and active pulmonary tuberculosis. We also measured the serum IL-4, IFN-γ, TGF-β and IL-10 levels to investigate the impact of regulatory T cells on the Th1 and Th2 immune status.
Sixty two patients with chronic asthma (Table 1) before treatment were recruited from the out-patient clinics of Soonchunhyang University Hospital. All of the patients exhibited one or more symptoms of asthma, and their physical examinations were compatible with the American Thoracic Society's definition of asthma (7). Each patient showed airway reversibility, as documented by a positive bronchodilator response of a greater than 15% increase in FEV1 and/or airway hyperreactivity to less than 10 mg/mL methacholine (8). Potential subjects who had less than a one-year duration of asthma, acute exacerbated asthma within four weeks, a history of brittle asthma, atopy to pollens, parenchymal lung disease apparent on chest radiography, diffusing capacity of less than 80%, previous inhaled steroid or systemic steroid use within the past four weeks, or maintenance theophylline or leukotriene antagonist therapies were excluded from the study.
Thirty-eight patients with pulmonary tuberculosis were enrolled in the study (Table 1). The patients had respiratory symptoms such as cough, sputum, fever, and chest discomfort. Pulmonary tuberculosis was diagnosed by chest roentgenography and a positive acid-fast bacilli (AFB) stain. None of the subjects took drugs, such as antihistamine, cromolyn, theophylline, or sympathomimetics, that could interfere with the performance of the skin tests within 72 hr of the tests. The study subjects were not allowed to consume coffee or tea on the day of the test. The blood samples were collected at the same time as the diagnosis was made.
A control group was used in the study, and all of the study and control group subjects had been vaccinated with BCG during infancy or childhood, which was confirmed by the presence of a vaccination scar. The study protocol was evaluated and approved by the Ethics Committee of Soonchunhyang University Hospital, and informed written consent was obtained from all of the study subjects.
Specimens of expectorated and induced sputa were collected from patients with suspected tuberculosis infection. The specimens were decontaminated, digested with N-acetyl-L-cysteine-NaOH (4%) for 15 min at room temperature, and neutralized with sterile 0.067 M phosphate-buffered saline (pH 6.8). All of the specimens were subjected to Ziehl-Nielsen staining for the detection of AFB.
Baseline measurements of FVC and FEV1 were selected according to the American Thoracic Society criteria (7). Basal and post-bronchodilator FEV1, FVC, and FEF25-75% were measured. Airway hyperreactivity was measured by methacholine challenge testing and expressed as the concentration required to cause a fall in the FEV1 of 20% (PC20) in noncumulative units.
Allergy skin prick tests were performed using commercially available inhalant allergens, which included dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus, Bencard Co., West Susses, U.K.) and histamine (1 mg/mL). None of the subjects had received antihistamines orally during the three days before the study. All of the tests included positive (1 mg/mL histamine) and negative (diluent) controls. After 15 min, the mean diameters of the wheals formed by the allergens were compared with the ones formed by histamine. If the former was the same or larger than the latter (A/H ratio≥1.0), the reaction was deemed positive. Atopy was determined by the presence of an immediate skin reaction to one or more aeroallergens as described previously (9).
An area of the skin on the volar surface of the forearm was cleaned with either acetone or ether. Two tuberculin units of polysorbate-stabilized purified protein derivatives (PPD) were injected intradermally into the volar surface of the forearm. The reactions were read at 48-72 hr by palpating the diameter of induration, which was measured in mm transversely to the long axis of the forearm. The diameter of erythema was not considered. If the diameter of the induration was larger than 10 mm, the reaction was deemed positive.
The serum levels of IL-4, IFN-γ IL-10, and TGF-β were quantified using a sandwich enzyme-linked immunosorbent assay kit according to the manufacturer's protocol (Biosource International Inc., San Diego, CA, U.S.A.). The lower limits of detection of IL-4, IFN-γ, IL-10, and TGF-β 1 were 7.8 pg/mL, 4.7 pg/mL, 7.8 pg/mL, and 62.5 pg/mL, respectively.
All data were analyzed using SPSS, version 7.5 for Windows. The data were expressed as mean±standard error of the mean (SEM). Mean values of measured variables were compared between the three groups using one-way analysis of variance. The relationships between cytokine levels were tested by Spearman product moment correlation. A p-value of <0.05 was considered significant.
The study included 62 patients with asthma, 38 patients with pulmonary tuberculosis before treatment, and 36 control subjects (Table 1). All of the patients with pulmonary tuberculosis had positive smears for AFB. The chest radiography abnormalities were located at the right upper lobe in nine cases, left upper lobe in 11 cases, right lower lobe in four cases, and multiple lobes in 14 cases. The PPD mean induration size was 22.7±11.4 mm (range, 0-43 mm). In the patients with tuberculosis, there were 24 cases of tuberculin reactors and six cases of atopy. The control group consisted of subjects with a negative allergy skin test and negative tuberculin response without any respiratory symptoms of tuberculosis or asthma.
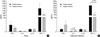
The level of IFN-γ was higher in patients with pulmonary tuberculosis than in those with asthma (30.3±10.56 pg/mL vs. 7.4±2.77 pg/mL, p=0.04, Table 2), but IL-4 was more prevalent in patients with asthma than in those with tuberculosis (15/62 vs. 2/38, p=0.01). The level of IFN-γ was higher in the pulmonary tuberculosis group than in the control group. The level of TGF-β1 was higher in the asthma group than in the tuberculosis and control groups (2977.9±824.2 pg/mL vs. 941.5±164.5 pg/mL vs. 1166.3±86.5 pg/mL, p<0.05, Table 2). The level of IL-10 was higher in the asthma and tuberculosis groups than in the control group (11.1±2.33 pg/mL vs. 16.4±3.22 pg/mL vs. 4.96±1.15 pg/mL, p<0.05, Table 2) but was similar between the asthma and tuberculosis groups. There was no relationship between atopy and the tuberculin response. The IFN-γ level correlated with the IL-10 level (r=0.353, p<0.05). Although there were no differences in the levels of IL-10 according to atopy, IL-10 was higher in the negative tuberculin reactors than in the positive tuberculin reactors among patients with asthma (Fig. 1). IL-4 was higher in the negative tuberculin reactors than in the positive tuberculin reactors among patients with tuberculosis (Fig. 2). TGF-β1 was higher in the positive tuberculin reactors than in the negative tuberculin reactors among patients with tuberculosis (Fig. 2).
The IL-10 level was higher in the negative tuberculin reactors than in the positive tuberculin reactors among patients with asthma in this study; conversely, the level of TGF-β1 was higher in the positive tuberculin reactors than in the negative tuberculin reactors among patients with tuberculosis. These results suggest that IL-10 and TGF-β may regulate asthma and pulmonary tuberculosis by Th2 and Th1 immune status and that the regulatory effects of circulating TGF-β and IL-10 on T cells may be different between Th2-type asthma and Th1-type tuberculosis.
The polarization of T-helper cells from T-helper 0 (Th0) cells is regulated by dendritic cells, the strength of the antigen stimulation, and the cytokines present during priming. T-helper subsets can be distinguished by their cytokine profiles. Th1 cells mainly produce IL-2 and IFN-γ, and Th2 cells mainly produce IL-4, IL-5, and IL-10. The same cytokines may also induce a shift in the balance between Th1 and Th2 responses. Through the release of particular cytokines, the subsets may mutually modulate the response. For instance, the release of IFN-γ by Th1 cells will inhibit the Th2 response, and the release of IL-10 by Th2 cells will inhibit the Th1 response (10). Th2 cells represent a polarized form of the T helper cell-mediated immune response characterized by the production of IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13, but not IFN-γ and tumor necrosis factor-β. By contrast, the other polarized form of the T helper cell response, which is defined as Th1, is characterized by the production of IL-2, IFN-γ, and TNF-β , but not the cytokines of the Th2-type response (11, 12). Asthma as an allergic disease develops early in life (13). Infections appear to afford a protection against the development of asthma and allergy (14), and children with eczema occasionally undergo temporary, spontaneous remission after severe bacterial and viral infections (15). Young adults who had experienced measles in childhood were significantly less likely to be atopic than those who had been vaccinated and/or had never had measles (16). The BCG vaccine is a potent immunoadjuvant for the induction of the Th1-type response and increases the production of IFN-γ (17). In this study, all of the patients had been vaccinated with BCG during infancy or childhood. As all of the patients in this study were adults, the effect of the BCG vaccine on the tuberculin response was minimal.
It has been hypothesized recently that infections involving a Th1 response will suppress the Th2 response based on the concept of the Th1/Th2 balance comprising the dichotomy in T helper cell populations through the mutual inhibition of the Th1 and Th2 cell subset. Infections of the Th1-type, such as Bordetella pertussis (18), Listeria monocytogenes (19), Mycobacterium bovis (20), Leishmania (21), and many others, induce an increased production of IFN-γ, which impairs the Th2 response and thus reduces the progression of allergy/asthma. Our findings that the IFN-γ level was higher in patients with pulmonary tuberculosis than in those with asthma and that IL-4 was more prevalent in patients with asthma than in those with tuberculosis suggest that asthma is a Th2-mediated disease and tuberculosis is a Th1-type disease. In our study, a relationship between atopy and the tuberculin response was not detected, which indicates that the Th1/Th2 paradigm is not relevant as the explanation of all asthma and tuberculosis phenotypes and that the Th1/Th2 paradigm cannot be the sole factor responsible for explaining the regulation of asthma and allergy.
After the exposure of mice to allergen by the respiratory route, regulatory CD4+ T cells developed, producing high levels of IL-10, which is typically considered to be a Th2 cytokine. The Tr cells down-modulated allergen-induced airway hyperreactivity in previously sensitized mice. Both the development and function of Tr cells depend on the presence of IL-10 and the interaction with an inducible co-stimulator expressed on dendritic cells. These dendritic cells also express B7-1 and B7-2. Akbari et al. (22) suggested that IL-10 may initially be involved in the polarization of Th2 responses and that it eventually plays a regulatory role late in immune responses to attenuate Th2-driven inflammatory activity. Although the level of IL-10 was higher in the asthma and tuberculosis groups than in the control group, there was no difference in the IL-10 level between the asthma and tuberculosis groups, suggesting that IL-10 may be important in the development of both asthma and tuberculosis, rather than only in the attenuation of Th2-driven inflammatory activity. Also, IL-10 levels correlated with IFN-γ, which suggests that IL-10 levels may mirror those of IFN-γ during the progression of airway diseases such as asthma and tuberculosis.
TGF-β is a pleiotropic cytokine produced by a variety of cells and exerts its effects depending on the effector cell and the context of production (23). The role of TGF-β signaling in T cells in immune regulation has been studied by impairing TGF-β signaling using dominant-negative TGF-β type II receptors (24, 25) or over-expression of Smad7, an inhibitory Smad protein (26). TGF-β acts on T cells to regulate airway reactivity and inflammation. Depending on the mode of immune stimulation, impaired TGF-β signaling in T cells modulates airway reactivity by the interaction of Th1 and Th2 cytokines (27).
The level of TGF-β1 was higher in the asthma group than in the tuberculosis and control groups, which indicates that TGF-β may be more involved in Th2-type asthma. The level of TGF-β1 was higher in positive tuberculin reactors than in negative tuberculin reactors among patients with tuberculosis, which regulate tuberculosis through a Th1 response.
The results presented in this study demonstrate that the regulatory effects of circulating TGF-β and IL-10 on T cell subsets may be different between Th2-type asthma and Th1 tuberculosis.
Figures and Tables
Fig. 1
Circulating cytokine profiles in patients with asthma by atopy (A) and tuberculin responses (B).
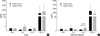
Fig. 2
Circulating cytokine profiles in patients with pulmonary tuberculosis by atopy (A) and tuberculin responses (B).
References
1. National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the diagnosis and management of asthma. 1997. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute;publication No: 97-4051A.
2. Chan J, Kaufman SH. Bloom BR, editor. Immune mechanisms of protection. Tuberculosis: Pathogenesis, protection and control. 1994. Washington: American society for microbiology press;389–415.

3. Shaheen SO. Changing patterns of childhood infection and the rise in allergic disease. Clin Exp Allergy. 1995. 25:1034–1037.
4. Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997. 275:77–79.

5. Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003. 15:627–633.

6. Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994. 265:1237–1240.

7. American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991. 144:1202–1218.
8. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease and asthma. Am Rev Respir Dis. 1987. 136:225–244.
9. Park CS, Kim YY, Kang SY. Collection between RAST and skin test for inhalant offending allergens. J Korean Soc Allergol. 1983. 3:1–9.
10. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998. 187:875–883.

11. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986. 136:2348–2357.
12. Del Prete GF, De Carli M, Mastromauro C, Bigiotti R, Macchia D, Falagiani P, Ricci M, Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen (s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991. 88:346–350.
13. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The group health medical associates. N Engl J Med. 1995. 332:133–138.
14. Holt PG, Sly PD, Bjorksten B. Atopic versus infectious diseases in childhood: a question of balance? Pediatr Allergy Immunol. 1997. 8:53–58.

15. Lacour M. Acute infections in atopic dermatitis: a clue for a pathogenic role of a Th1/Th2 imbalance? Dermatology. 1994. 188:255–257.

16. Shaheen SO, Aaby P, Hall AJ, Barker DJ, Heyes CB, Shiell AW, Goudiaby A. Cell mediated immunity after measles in Guinea-Bissau: historical cohort study. Br Med J. 1996. 313:969–974.

17. Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.

18. Mills KH, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993. 61:399–410.

19. Yeung VP, Gieni RS, Umetsu DT, Dekruyff RH. Heat-killed Listeria monocytogenes as an adjuvant converts established murine Th2-dominated immune responses into Th1-dominated responses. J Immunol. 1998. 161:4146–4152.
20. Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993. 178:567–577.

21. Schlaak JF, Nieder P, Meyer zum Buschenfelde KH, Fleisher B. Human T helper cells reactive with somatic bacterial antigens belong to the Th1 subset. Med Microbiol Immunol Berl. 1994. 183:169–175.

22. Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002. 8:1024–1032.

23. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998. 16:137–161.

24. Gorelik L, Flavell RA. Abrogation of TGF-β signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000. 12:171–181.

25. Lucas PJ, Kim SJ, Melby SJ, Gress RE. Disruption of T cell homeostasis in mice expressing a T cell-specific dominant negative transforming growth factor-β II receptor. J Exp Med. 2000. 191:1187–1196.
26. Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, Iwamoto I. Blockade of transforming growth factor β/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000. 192:151–158.

27. Schramm C, Herz U, Podlech J, Protschka M, Finotto S, Reddehase MJ, Kohler H, Galle PR, Lohse AW, Blessing M. TGF-βII regulates airway responses via T cells. J Immunol. 2003. 170:1313–1319.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download