Abstract
Angiogenesis, formation of new microvessels providing oxygen and nutrient supply, is essential for tumor growth. It is dependent on the production of angiogenic growth factors by tumor cells. Angiopoietin 1 (Ang-1) and 2 (Ang-2) and their common receptor, Tie2, are thought to be critical regulators of tumor angiogenesis. We examined expression of Ang-1, Ang-2, and their common receptor Tie2 mRNAs and proteins in gastric cancers using in situ hybridization and immunohistochemistry. We also investigated the relationship between their expression and differentiation of cancer cells, lymph node metastasis, tumor size, depth of cancer cell invasion, TNM staging and microvessel density (MVD). The expression of Ang-1, Ang-2, and Tie2 mRNA in cancer cells significantly correlated with the MVD (p<0.001, <0.001 and =0.019, respectively). Ang-1 and Tie2 positivity correlated with advanced gastric cancers (p<0.05) and larger cancers had higher positive rates of Ang-1, Ang-2, and Tie2 mRNA expression (p<0.001, =0.010 and =0.039, respectively). Significant positive correlations were also found between mRNA expression of Tie2 and those of Ang-1 and Ang-2 (p<0.01 and <0.001, respectively). These findings indicate that the expression of Ang-1 and Ang-2 is important for tumor angiogenesis, and suggest a possible role of autocrine/paracrine function of angiopoietin/Tie2 system in gastric cancer progression.
Angiogenesis, generation of new microvessels from preexisting blood vessels, is essential for tumor growth and invasion (1). Cancer cells stimulate angiogenesis by secreting angiogenic growth factors and cytokines, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and fibroblast growth factor (FGF) that act on the endothelial cells of adjacent vessels and microvessels (2). Angiopoietins, a new family of angiogenic growth factors that are mostly specific for the vascular endothelium, have been identified in recent years (3-5). Angiopoietins have been shown to function as ligands for the Tie2/Tek vascular endothelial-specific receptor (6, 7). Angiopoietin 1 (Ang-1) functions to stabilize and maintain mature vessels by promoting interaction between endothelial cells and its supporting cells such as pericytes and smooth muscle cells (8, 9). Angiopoietin 2 (Ang-2) is expressed at sites of vascular remodeling and is thought to play a facilitating role at sites of vascular remodeling by disrupting the constitutive stabilizing action of Ang-1 (5).
Gastric cancer is the second most common malignancy in the world. It has been demonstrated that certain cancer cells (e.g. breast, stomach) produce several angiogenic growth factors, including VEGF, PDGF and transforming growth factor β1 (TGF-β1) and the expression of these factors correlate with tumor angiogenesis, tumor progression and poor prognosis (2, 10-12). Among the known angiogenic factors, VEGF has emerged as the central regulator of the angiogenic process in cancer (2). Increased expression of VEGF in gastric cancer has been demonstrated and it is correlated with tumor angiogenesis and poor prognosis (11-13). However, little is known about the expression of angiopoietins and Tie2 in gastric carcinoma and their relation to angiogenesis and clinicopathologic findings. Angiopoietin receptors, Tie2/Tek, were previously thought to be expressed exclusively by endothelial cells (6, 7). Recently, some studies have suggested that Tie2 could be expressed in hematopoietic precursors and cancer cells (14, 15) and prostate carcinoma cells (16). However, the possibility that angiopoietins/Tie2 are expressed in gastric cancer and can function in an autocrine or paracrine manner has not been previously examined.
In the present study we examined the expression and localization of Ang-1 and Ang-2 mRNAs and proteins in human gastric carcinomas and investigated the correlation between angiogenesis and differentiation of carcinomas, lymph node metastasis, tumor size, depth of invasion, and TNM staging.
The Human Ethics Committee of Chonbuk National University Medical School approved this study. We used gastric cancer specimens obtained from 51 patients (between 1998 and 1999) at Chonbuk National University Hospital who underwent curative gastrectomy without prior chemotherapy or radiation therapy. There were 35 male patients and 16 female patients with ages ranging from 34 to 76 yr (mean, 60.2 yr). Clinicopathologic data obtained included histological type of gastric cancer, differentiation, lymph node metastasis, size of tumor, and post-operative TNM staging. The pathologic findings were determined according to guidelines established by the Japanese Society Committee on Histological Classification of Gastric Cancer (17). The TNM staging was determined based on criteria of the American Joint Committee on Cancer (AJCC) (18). The cases have included 38 tubular adenocarcinomas and 13 signet ring cell carcinomas. In 38 cases diagnosed as tubular adenocarcinoma, there were 2 cases of well differentiated, 18 cases of moderately differentiated and 18 cases of poorly differentiated adenocarcinoma.
Tissue detection of the mRNAs for human Ang-1, Ang-2, and Tie2 were performed using in situ hybridization. Paraffin-embedded sections and digoxigenin-labeled sense and anti-sense RNA probes were used. The human Ang-1, Ang-2, and Tie2 RNA probes were generated from linearized pBluescript II KS+ plasmid (Stratagene, La Jolla, CA, U.S.A.), which contains an Hind III-Eco RI fragment corresponding to nucleotides 396 through 809 of the human angiopoietin-1 cDNA, a Xho I-Bam HI fragment corresponding to nucleotides 638 through 920 of the human angiopoietin-2 cDNA, and an Hind III-Xbal fragment corresponding to nucleotides 511 through 668 of the human Tie2 cDNA, respectively. Digoxigenin-labeled RNAs were synthesized using a DIG RNA Labeling kit (Boehringer Mannheim, Indianapolis, IN, U.S.A.). Sections were preincubated in mRNA in situ hybridization solution (DAKO, Carpinteria, CA, U.S.A.). Hybridization was carried out overnight at 55℃ in a humidified chamber. The concentration of hybridization mixture was 0.5 ng RNA probe per 1 µL of mRNA in situ hybridization solution used for the prehybridization step. Posthybridization wash was performed at 53℃ in 0.1×stringent wash solution (DAKO, Carpinteria, CA, U.S.A.). After blocking the nonspecific protein binding with Protein Block Serum-Free (DAKO), the mRNA signals were detected using anti-digoxigenin/alkaline phosphatase (DIG/AP) antibody and bromochloroindoylphosphate/nitrobluetetrazolium (BCIP/NBT) chromogen substrate (DAKO). Sense probes were used as negative controls and staining evaluation was performed under the same conditions. The extent of angiopoietin and Tie2 staining were recorded using a grading system, based on the percentage of cancer cells positively stained: grade 0=0-10% cells; grade 1=11-70% cells; grade 2=>70% cells positively stained. When the positive cells were more than 10%, we regarded it as positive.
For immunohistochemical staining, the immunoperoxidase method was used with the streptoavidin-biotinylated horseradish peroxidase complex (DAKO). Four µm thick sections were cut from the formalin-fixed and paraffin-embedded tissue blocks. For angiopoietin 1 and angiopoietin 2 immunostaining, sections were treated with target retrieval solution (DAKO) for 20 min at 97℃, and then incubated in methanol containing 0.3% hydrogen peroxide at room temperature for 20 min to block endogenous peroxidase. Subsequently, sections were incubated with Protein Block Serum-Free (DAKO) at room temperature for 10 min and were then incubated for 2 hr at room temperature with anti-factor VIII related antigen antibody (DAKO), which stains only endothelial cells, or overnight at 4℃ with anti-angiopoietin 1, 2 or Tie2 (Chemicon International, Temecula, CA, U.S.A.) primary antibodies. After washing, the sections were incubated with a biotin-conjugated secondary antibody at room temperature for 30 min and finally with peroxidase conjugated streptoavidin at room temperature for 30 min. Peroxidase activity was detected with the enzyme substrate 3 amino-9-ethyl carbazole. Sections treated the same way described above, except they were incubated with Tris buffered saline instead of the primary antibody, served as the negative controls.
Sections stained for factor VIII related antigen, which visualizes endothelial cells, were used for determination of microvessel density (MVD). Sections were screened under ×40 magnification to identify the areas with the highest vascular density within the tumor. Microvessels were counted in 4 areas under ×200 magnification. Any single stained cells or cluster of endothelial cells that were clearly separated from adjacent microvessels, tumor cells, and other connective tissue elements were considered as vessels.
The relationship between expression of Ang-1, Ang-2, and Tie2 mRNA, and microvessel density was analyzed using Student's t-test. Associations between the expression of Ang-1, Ang-2, and Tie2 mRNA, and clinicopathologic factors were tested by chi-square test. The following clinicopathologic factors were correlated with angiogenic factor expression: age, sex, differentiation of cancer cells (differentiated; well and moderately differentiated carcinomas vs. undifferentiated; poorly differentiated and signet ring cell carcinomas), tumor depth (early gastric cancer [Tis, carcinoma in situ; T1, tumor invades lamina propria or submucosa] vs. advanced gastric cancer {T2, tumor invades the muscularis propria or the subserosa; T3, tumor penetrates the serosa without invading adjacent structures; T4, tumor invades adjacent structures]), lymph node metastasis, size of tumor (<2 cm vs. ≥2 cm) (19), and post-operative TNM staging (I+II vs. III+IV). A p-value of less than 0.05 was considered significant.
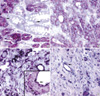
Ang-1 mRNA was expressed in 30 of 51 specimens (58%); 9 tumors were grade 1 (11-70% cells positively stained), and 21 were grade 2 (>70% cells positively stained). Ang-2 mRNA was expressed in 25 of 51 specimens (49%); 8 tumors were grade 1, and 17 were grade 2. Tie2 mRNA was expressed in 10 of 51 specimens (19%); 7 were grade 1, and 3 were grade 2. All ten Tie2 mRNA positive specimens expressed Ang-1 mRNA, Ang-2 mRNA or both. Ang-1 and Ang-2 mRNA were mainly expressed in cancer cells as a strong cytoplasmic staining (Fig. 1A, B). No or minimal staining was observed in normal and metaplastic gastric mucosal cells (Fig. 1A). In addition to the strong staining present in carcinoma cells, smooth muscle cells of large vessels, occasional stromal cells and endothelial cells demonstrated positive staining for Ang-1 mRNA (Fig. 1C). Occasionally, endothelial cells of blood vessels expressed Ang-2 mRNA. Tie-2 mRNA was mainly expressed in infiltrating cancer cells of undifferentiated group and in endothelial cells (Fig. 1D, E). Expression of Tie2 mRNA was predominantly confined to T2-4 classification and carcinomas of undifferentiated group. No specific staining was present when the sense probes were used. We selected the 10, 10, and 5 representative gastric carcinoma specimens with a strong Ang-1, Ang-2 and Tie2 mRNA expression and performed immunostaining for Ang-1, Ang-2 and Tie2 proteins in order to compare localization of proteins and mRNAs. Immunostaining with polyclonal antibodies specific for human Ang-1, Ang-2 and Tie2 showed that localization of these proteins and respective Ang-1, Ang-2 and Tie2 mRNA was very similar in all specimens (Fig. 1F-H).
The microvessel counts in gastric cancer specimens ranged from 5 to 71 with a mean value of 27.7 (standard deviation, 16.3). Ang-1, Ang-2 and Tie2 mRNA expression significantly correlated with the MVD. Table 1 shows the correlation between MVD and Ang-1, Ang-2 and Tie2 mRNA expression. Large gastric cancers (≥2 cm) had a significantly higher positive rate of Ang-1, Ang-2 and Tie2 mRNA expression than small-sized (<2 cm) ones (Table 2). The Ang-1 and Tie 2 positive rates were also higher in T2-4 cancers than those of Tis and T1 cancers. A strong correlation was found between Ang-1 and Ang-2, and Tie2 mRNA expression (p=0.002, and p<0.001, respectively). There was no close correlation between the expression of these angiogenic factors and sex, age, tumor stage, histologic types of cancer, and lymph node metastasis. Although there was a tendency for higher Tie2 mRNA expression in carcinomas of undifferentiated group compared with the carcinomas of differentiated group, this correlation was not statistically significant (p=0.165).
In the majority of cancers, tumor growth is critically dependent on blood vessels because of the requirement for increased nutrient and oxygen supply. Cancer cells can induce the formation of new blood vessels from pre-existing ones through the process of angiogenesis (1, 2). This process is controlled by several angiogenic factors, including acidic and basic FGF, VEGF, PDGF, TGF, and angiopoietins (2-5, 8-13). Ang-1 and its naturally occurring antagonist, Ang-2, regulate tyrosine phosphorylation of Tie2/Tek receptors on endothelial cells (3-9). Several studies have demonstrated the important role of angiopoietins as a mediator of angiogenesis in various tumors, such as breast, colon, brain, liver and lung cancers (20-27). In gastric cancer, the relationship between expressions of angiopoietin and angiogenesis, or clinicopathologic findings has not been fully examined.
Our study showed that the proportion of gastric cancers expressing Ang-1, Ang-2, and Tie2 is significantly higher in advanced cancers, and in larger size tumors. Furthermore, expression of these angiogenic factors significantly correlated with tumor MVD. Our findings are consistent with the result of Etoh et al. (28), who reported that Ang-2 mRNA levels correlated with more advanced stages and more frequent vascular involvement in gastric cancer. In other types of cancer, for example, brain, liver, and lung cancer, high expression of Ang-1 and Ang-2 correlated positively with tumor angiogenesis and tumor growth (22, 24, 25, 27, 29). However, this issue remains somewhat controversial. Hayes et al. reported that in breast cancer overexpression of Ang-1 did not enhance tumor growth (20). Another study showed a significant reduction in expression of angiopoietins in breast cancers compared with that of normal breast tissue (26). Our present data support the contention that angiopoietins and their receptor Tie2 play an important role in gastric cancer angiogenesis and cancer growth.
While the role of Tie2 receptor and angiopoietins in developmental angiogenesis has been intensively studied, little is known about their expression and function in malignant cells. Although the precise function of Tie2 expressed in tumor cells remains unclear, our findings of Tie2 expression in gastric cancer cells is particularly interesting in light of recent observations that Tie2 can be expressed by certain types of tumor cells, hematopoietic, prostate cancer and giant cell tumors of tendon sheath (14-16, 30). Certain angiogenic factors, such as FGF-1 and VEGF, have receptors distributed not only in tumor cells, but also in surrounding stromal cells and endothelial cells of vessels. This distribution suggests the possible paracrine or autocrine regulation of tumor growth by angiogenic factors (10, 31). In our present study, some specimens showed that cancer cells, as well as endothelial cells, express Tie2. The expression of Tie2 was significantly associated with large size tumors, advanced gastric cancer, and increased MVD. Moreover, a strong correlation was found between Ang-1, Ang-2, and Tie2 mRNA expression. Emerging evidence suggests that the autocrine activity of VEGF could be important for tumor cell survival, and growth (10, 32, 33). Recently, Soker et al. (32) reported that the expression of VEGF receptors by prostate tumor cells correlates with progression to a more malignant phenotype, and increased chemotactic migration of FB2 prostate tumor cells, and suggested an autocrine signaling loop involving VEGF and its receptor. Our findings are consistent with the recent paper of Nakayama et al. (34), who reported that Tie receptors and angiopoietins were highly expressed in human gastric adenocarcinoma cells and the Tie-Ang receptor-ligand complex is one of the factors involved in the progression of human gastric adenocarcinoma. In our study, we found that Tie2 expression was mainly confined to advanced, undifferentiated carcinomas. While a functional role for Tie2 receptor expression in tumor cells has not been reported, our observation suggests that the Tie2 receptor may be related to tumor progression or dedifferentiation of gastric cancer cells. Further analysis of Tie2 expression by cancer cells is required to determine its mechanism of action and whether Tie2 has an important role in gastric cancer progression.
In summary, our findings indicate that expression of Ang-1 and Ang-2 is associated with and most likely related to increased angiogenesis and tumor growth in human gastric cancers. The expression of both angiopoietins and their Tie2 receptor in gastric cancer cells suggests a possible autocrine/paracrine regulation of gastric cancer cell growth and may be involved in the emergence of an aggressive phenotype during gastric cancer progression.
Figures and Tables
Fig. 1
(A-E) In situ hybridization for Ang-1, Ang-2 and Tie2 mRNA. (A) Positive Ang-2 expression in well-differentiated cancer cells (arrowheads) with no or only minimal staining on adjacent metaplastic gastric mucosa (arrows) (×100). (B) Moderately differentiated cancer cells show intense cytoplasmic staining for Ang-2 mRNA (×200). (C) Positive Ang-1 mRNA signals on cancer cells (arrowheads, ×100) as well as smooth muscle cells and endothelial cells of large vessels (arrows, Inset, ×400). (D) Poorly differentiated cancer cells (arrowheads) and endothelial cells (arrow) show Tie2 mRNA expression (×100). (E) Infiltrating poorly differentiated carcinoma cells of the muscle layer (arrowheads, ×100) show positive cytoplasmic Tie2 mRNA expression (Inset, ×400). (F-H) Immunohistochemistry for Ang-1, Ang-2 and Tie2 protein. (F) Moderately differentiated cancer cells exhibit Ang-2 protein expression (×200). (G) Immunoreactivity for Ang-1 on smooth muscle cells of vessels (arrows), stromal cells and cancer cells (arrowheads) (×200). (H) Immunohistochemistry for Tie 2. Same cancer area to Fig. E (×100) shows positive cytoplasmic expression on tumor cells (Inset, ×400).
References
1. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.

2. Fox SB, Gatter KC, Harris AL. Tumor angiogenesis. J Pathol. 1996. 179:232–237.
3. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996. 87:1161–1169.

4. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996. 87:1171–1180.

5. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997. 277:55–60.

6. Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene. 1993. 8:1293–1301.
7. Runting AS, Stacker SA, Wilks AF. Tie2, a putative protein tyrosine kinase from a new class of cell surface receptor. Growth Factors. 1993. 9:99–105.
8. Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998. 282:468–471.

9. Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999. 79:213–223.
10. Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast cancer cells. Cancer Res. 2001. 61:5736–5740.
11. Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The expression of transforming growth factor-β1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999. 86:1455–1462.

12. Maeda K, Kang SM, Ogawa M, Onoda N, Sawada T, Nakata B, Kato Y, Chung YS, Sowa M. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial cell growth factor expression in gastric carcinoma. Int J Cancer. 1997. 74:545–550.

13. Maeda K, Kang SM, Onoda N, Ogawa M, Sawada T, Nakata B, Kato Y, Chung YS, Sowa M. Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology. 1998. 55:594–599.

14. Shaw JP, Basch R, Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004. 32:168–175.

15. Muller A, Lange K, Gaiser T, Hofmann M, Bartels H, Feller AC, Merz H. Expression of angiopoietin-1 and its receptor TEK in hematopoietic cells from patients with myeloid leukemia. Leuk Res. 2002. 26:163–168.
16. Wurmbach JH, Hammerer P, Sevinc S, Huland H, Ergun S. The expression of angiopoietins and their receptor Tie-2 in human prostate carcinoma. Anticancer Res. 2000. 20:5217–5220.
17. Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. 1995. Tokyo: Kanehara & Co. Ltd..
18. American Joint Committee on Cancer. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, editors. Stomach. AJCC cancer staging manual. 2002. 6th ed. New York, NY: Springer Publishers;99–103.
19. Fenoglio CM, Noffsinger AE, Stemmermass GN, Lantz PE, Listrom MB, Rilke FO, editors. The Neoplastic Stomach. Gastrointestinal Pathology An Atlas and Text. 1999. 2nd ed. Philadelphia, NY: Lippincott-Raven Publishers;265.
20. Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000. 83:1154–1160.

21. Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001. 61:1255–1259.
22. Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004. 165:1557–1570.

23. Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R, Sata M, Nakashima O, Yano H, Kojiro M. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004. 40:799–807.

24. Koga K, Todaka T, Morioka M, Hamada J, Kai Y, Yano S, Okamura A, Takakura N, Suda T, Ushio Y. Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res. 2001. 61:6248–6254.
25. Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003. 16:552–557.

26. Currie MJ, Gunningham SP, Han C, Scott PA, Robinson BA, Harris AL, Fox SB. Angiopoietin-1 is inversely related to thymidine phosphorylase expression in human breast cancer, indicating a role in vascular remodeling. Clin Cancer Res. 2001. 7:918–927.
27. Tanaka F, Ishikawa S, Yanagihara K, Miyahara R, Kawano Y, Li M, Otake Y, Wada H. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002. 62:7124–7129.
28. Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001. 61:2145–2153.
29. Shim WS, Teh M, Mack PO, Ge R. Inhibition of angiopoietin-1 expression in tumor cells by an antisense RNA approach inhibited xenograft tumor growth in immunodeficient mice. Int J Cancer. 2001. 94:6–15.

30. Nakashima M, Uchida T, Tsukazaki T, Hamanaka Y, Fukuda E, Ito M, Sekine I. Expression of tyrosine kinase receptors Tie-1 and Tie-2 in giant cell tumor of the tendon sheath: a possible role in synovial proliferation. Pathol Res Pract. 2001. 197:101–107.

31. Lehtola L, Partanen J, Sistonen L, Korhonen J, Warri A, Harkonen P, Clarke R, Alitalo K. Analysis of tyrosine kinase mRNAs including four FGF receptor mRNAs expressed in MCF-7 breast-cancer cells. Int J Cancer. 1992. 50:598–603.

32. Soker S, Kaefer M, Johnson M, Klagsbrun M, Atala A, Freeman MR. Vascular endothelial growth factor-mediated autocrine stimulation of prostate tumor cells coincides with progression to a malignant phenotype. Am J Pathol. 2001. 159:651–659.

33. Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, Hicklin DJ, Witte L, Moore MA, Rafii S. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000. 106:511–521.

34. Nakayama T, Yoshizaki A, Kawahara N, Ohtsuru A, Wen CY, Fukuda E, Nakashima M, Sekine I. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohistochemical analyses and correlation with clinicopathologic factors. Histopathology. 2004. 44:232–239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download